1. INTRODUCTION
Regenerative medicine represents a coming revolution in the treatment of many illnesses. Emerging therapies that use stem cells harvested from the patients themselves are demonstrating extremely promising results in clinical trials and other studies. Referred to as stem-cell therapies, or just cell therapies, they involve taking stem cells from the body, culturing them, and then putting them back into the body to induce tissue to regrow. Japanese researchers are world leaders in this field and their many years of hard work are bearing fruit as clinical trials progress according to schedule. We can be optimistic that novel therapies will soon make currently untreatable diseases and disorders treatable.
The chapters of this collection describe six regenerative therapies. Before considering specifics, however, in this essay I provide an overview, explaining the principles common to all regenerative therapies: the use of bioactive stem cells in a patient and the provision of framework materials as scaffolds for tissue regeneration using tissue engineering.
2. WHAT IS STEM-CELL THERAPY?
Stem cells are found in various tissues in the body, including the blood, bone marrow, fat, connective tissue, nerves, skin, etc. When stimulated, stem cells have the capacity to produce specific, mature cell types. Stem cells are believed to replenish damaged or dead cells in the body.
The stem cell therapies described here use adult stem cells. Since cell manipulation is not applied, there is no risk of developing tumours. The ethical issues associated with embryonic cells are also avoided. There is no risk of rejection by the immune system, as the patient’s own cells or immunotolerant stem cells are used. This means that, unlike organ transplantation, stem-cell therapy does not need adjuvant therapies such as the continuous administration of immunosuppressants. Furthermore, both cell extraction from the body and cell replacement (either as-is or after culturing) can be partially automated. Many procedures can be performed using relatively simple techniques, such as intravenous infusion and other techniques that do not require general anaesthetic.
Adult stem cells fall into two broad categories. One is haematopoietic stem cells. As their name indicates, these stem cells can produce blood cells, but they can also generate vascular cells. The other type is currently referred to as mesenchymal stem cells. They are so named because these stem cells were originally thought to produce cells that originate in the mesoderm (mesenchyme), including the bone and fat. However, recent studies have revealed that some mesenchymal stem cells can create nerve cells, which are not of mesodermal origin. Further research is needed to uncover more about these cells.
How do we identify adult stem cells when they exist only in minute quantities in the body? The answer lies in using markers for the glycoproteins that are expressed on their membranes. For example, since haematopoietic stem cells have a glycoprotein called CD34, they can be identified as CD34+ cells. These CD34+ haematopoietic stem cells show potential for treating blood vessels in the legs that have been obstructed in critical limb ischemia (Stem Cell Therapy 3) and for treating intractable bone fractures (Tissue Engineering 1) in combination with a scaffold. An example of an emerging stem-cell therapy that uses mesenchymal stem cells is one for spinal cord injuries (Stem Cell Therapy 1), in which nerve cells are regenerated using CD105+ cells.
Some adult stem cells defy categorization due to their diverse characteristics. For example, multilineage differentiating stress enduring (Muse) cells, which were discovered by Mari Dezawa of Tohoku University in 20101, are thought to create cells of various tissue types and to play a specialized role in the repair of body tissues. Muse cells are the basis for an translational approach to treating myocardial infarction (Stem Cell Therapy 2) and have been used to regenerate cardiac muscles, which had previously been considered difficult to do. Remarkably, studies have indicated that intravenous infusion of Muse cells is effective for treating myocardial infarction patients and, despite being an allogeneic transplant, immunosuppression is not needed for the initial infusion.
The above-mentioned stem-cell approaches all employ extremely simple medical procedures. They harness the body’s innate healing mechanism, by extracting stem cells, boosting them outside the body and then returning them to the patient, as is the case in intravenous infusion of stem cells from the patient. The therapies are based on biological principles known as stem-cell physiology, which have been described in the publications listed in Ref. 2. The basic steps of this mechanism are tissue damage → signalling → signal perception by stem cells → migration → homing → conditioning → repair. Stem cells are found throughout the body, and they maintain the processes of life. But, when the need arises, stem cells are mobilized from the bone marrow and home in on the site requiring repair, where they enter the lesion to condition and conduct the repair process. This is the essence of the morphological functional homeostatic mechanism of living organisms, which stem-cell therapies harness. In conjunction with this, regenerative medicine also uses cells that are not stem cells per se but can be activated either to migrate to the lesion (for example, cells at the periphery of the eardrum, which are induced by basic fibroblast growth factor; Tissue Engineering 3) or to self-renew (for example, oral mucosa cells; Tissue Engineering 2). Tissue engineering is the key to the success of therapies that use these cells (Fig. 1).
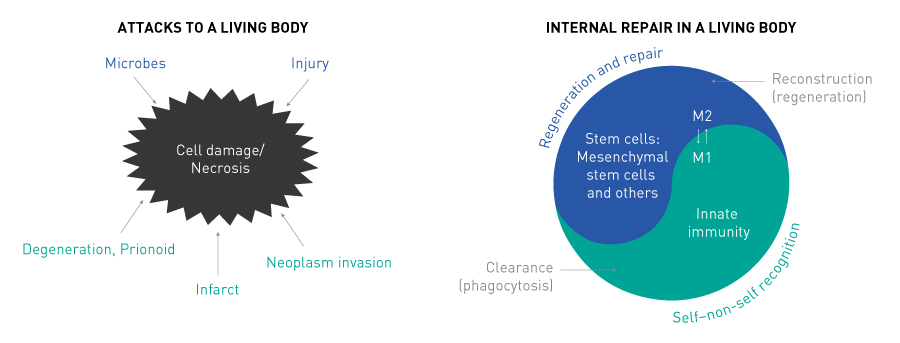
Multicellular symbiotic system for maintaining homeostasis that inherits self-preserving ability
© 2019 TRI
3. PROVIDING SCAFFOLDS THROUGH TISSUE ENGINEERING
When a damaged area of a tissue has a gap or hole, scaffolds are needed for cells to grow on. This is the purview of tissue engineering, and some of its elemental techniques have already been commercialized. It is vital to appropriately combine cellular regenerative medicine with tissue engineering techniques.
A diverse range of tissue engineering techniques are available. For example, a gelatin sponge called Spongel® is used in the regeneration of the eardrum (Tissue Engineering 3). Eardrum cells can be grown by stimulating the basic fibroblast growth factor. To activate the cells, Spongel® is used to plug a hole in the eardrum. Since the eardrum is not flat, a three-dimensional approach is needed, which is why Spongel® is used to fill a perforation in the tympanic membrane. A coating of fibrin adhesive is then applied to cause the regenerative cells to migrate from the periphery of the eardrum to the Spongel® scaffold — this is what makes the therapy effective. Remarkably, a natural three-layered membrane forms inside the Spongel® scaffold.
Investigational approaches to regenerating the cornea in the eye (Tissue Engineering 2) involves culturing a sheet of oral mucosa cells on amnion membrane. The damaged area of the cornea is cut away, and the cultured cell sheet is placed on the area. In proposed treatments for intractable fractures (Tissue Engineering 1), CD34+ cells and collagen are injected together into the non-union area of fractured bone. A fibrous protein known as atelocollagen is used to fill in the fractured part of the bone and form a scaffold for bone cell regrowth.
4. TREATMENT DESIGN BASED ON CELL PRINCIPLES
Research conducted globally on regenerative therapies for various diseases has not always been successful. How have Japanese researchers achieved a series of good outcomes? One reason is that they design therapeutic approaches based on a deep understanding of biological processes that occur in the human body.
Living organisms maintain homeostasis through mutual interactions between different cells. If some abnormality occurs in the body, it is detected and stem cells are mobilized to the site, where they start to address the abnormality. This cell activity includes discriminating between cells that should be allowed to live and those that should die. It then involves sending in the necessary factors to cells designated for preservation, and eliminating inflammation or promoting blood-vessel development. New cells are regenerated when the tissue has been repaired.
Cells function in a similar way to human society, performing operations in an ordered sequence. Stem cells may be regarded as leaders in this process, giving instructions to other cells. For major traumas, there are insufficient stem cells naturally present in the human body, and hence stem cells need to be cultured in vitro to multiply their number and make up for what the body cannot provide. When designing therapies, it is thus important to clearly identify what is happening in a specific disease or disorder as well as what is missing.
For example, stem cells can be administered intravenously to the body or by local administration to the lesion. Intravenous administration gives good outcomes when treating spinal injuries and myocardial infarction; this is thought to be because the stem cells used are those normally involved in repairing and regenerating the peripheral microcirculatory system.
Designing a therapy requires understanding the biological principles and cell processes involved in maintaining the homeostatic health of multicellular organisms. It is vital to consider how such organisms preserve integrity, maintain homeostasis, form tissues and scaffolds. It also important to know about other aspects such as self-organizational principles, cell symbiosis, rhythm, synchronism and symmetry. These principles all orchestrate processes in living organisms and are overall encompassing activities. Keeping these principles in mind, we then need a full understanding of the similarity between animal models and human disorders; in other words, how animal models mirror the type, pathology, state and stage of a human disorder.
Therapies should be developed and evaluated by conducting registration trials that comply with the law with a view to filing for licensing. When conducting a registration trial, exact data satisfying the strict criteria of good manufacturing practice (GMP), good laboratory practice (GLP) and good clinical practice (GCP) standards can be obtained through the guidance and advice offered by the Pharmaceuticals and Medical Devices Agency (PMDA; the Japanese equivalent of the FDA in the United States). In Japan, the Ministry of Health, Labour and Welfare instituted the Sakigake Designation System in 20153, which allows therapies developed in Japan that show strong promise but that have not been approved in other countries to be fast tracked in the authorization process and to be prioritized in screening and evaluation. Therefore, applying for licensing under this system is highly recommended.
5. TRANSFORMATION OF MEDICAL CARE
This collection of articles focuses on disorders resulting from damage to six types of tissue: nerve, vascular, myocardial, bone, eardrum and corneal. New therapies for more-complex diseases will be reported in the future. Promising targets for future therapies include conditions where the autoimmune system attacks tissues, such as ulcerative colitis and hepatic cirrhosis, and diseases where prionoids attack nerve cells, such as Alzheimer’s disease. To deal with such diseases, our existing knowledge of cell mechanisms needs to be supplemented by forthcoming therapeutic principles gained from the stem-cell therapies and tissue-engineering technologies presented in this collection. However, practical treatments for these diseases are not far off. We also envisage developing ways to overcome arteriosclerosis and major cancers, such as prostate and bowel cancers. We have already reported on new treatments for ulcerative colitis in a white paper associated with a recent Nature Outline. In the future, we hope to be able to report on the next stages of regenerative medicine, such as one-step methods for adjusting the quantity of stem cells required for therapy, the use of new biologically active materials produced by stem cells (but that does not require using stem cells), and the development of drugs that mobilize or activate stem cells in vivo.
Regenerative medicine based on stem-cell therapy and tissue engineering promises to provide definitive therapeutic outcomes for various diseases and disorders by applying relatively simple procedures. As such, regenerative medicine has rendered obsolete the accepted norms of existing drug-development strategies, and it demands huge innovations in the concepts of drug therapy and drug discovery. Regenerative medicine will be fundamental for future medical care around the globe. As the development of regenerative medicine enters its second exciting stage, we are certain this is where the future of medicine lies.
References
- Kuroda, Y., Kitada, M., Wakao, S., Nishikawa, K., Tanimura, Y. et al. Unique multipotent cells in adult human mesenchymal cell populations. Proceedings of the National Academy of Sciences USA 107, 8639–8643 (2010) | article
- For Nature supplements that highlight research conducted by researchers associated with TRI, see TRI Advances website. See also Nature Outlook articles
- Sakigake Designation System (Japanese only), Ministry of Health, Labour and Welfare website | article