Author affiliations: Translational Research Center for Medical Innovation, Institute for Biomedical Research and Innovation at Kobe, Japan.
A new treatment has been developed by researchers associated with the Translational Research Center for Medical Innovation (TRI) in Japan that involves stimulating tissue stem cells or progenitor cells in order to repair perforations in the tympanic membrane. This treatment is safer and easier to administer than currently available therapies. It involves enlarging perforations and providing a gelatin sponge impregnated with basic fibroblast growth factor as a substrate for new cells to grow on. The Japanese government approved the treatment in August 2019, making it the first approved tissue-engineering treatment in Japan for repairing perforations in the tympanic membrane.
1. INTRODUCTION
Regenerative medicine seeks to harness the natural process of regeneration by inducing cells to differentiate and proliferate so that they reconstruct irreversibly damaged tissues and organs.
A fundamental property of living organisms is their ability to restore damaged components. The extent of restoration depends on the severity of the damage. In cases of fairly minor damage, tissues or organs can spontaneously heal through regeneration, being completely restored to their original state. In more severe cases, healing may fail to restore the original state, resulting in deformation or degeneration. The innate regenerative capacity also varies with the tissue or the organ. For example, even when cut in half, the liver can regenerate to almost its original size within a few months. Regenerative capacity also varies with species. For instance, newts and lizards can regrow severed tails and limbs. Generally, tissues and organs with active cell division, and lower forms of life, have a high regenerative capacity. Research into developmental processes and regeneration in various animal species has led to the development of regenerative medicine, which seeks to find ways to restore tissues and organs to their original state by promoting non-spontaneous regeneration.
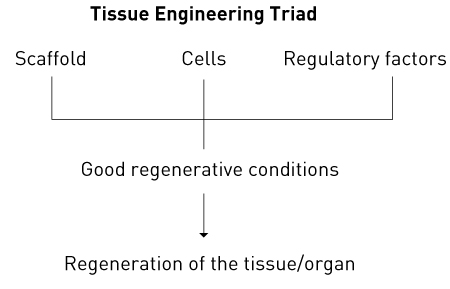
Figure 1. The tissue engineering triad.
Regenerative medicine has its origins in the artificial-organ research conducted in the 1950s, mainly in Europe and the United States. Materials with a high affinity for tissues were studied and developed with a view to implanting artificial organs in the body, a contrasting approach to ex vivo technologies such as dialysis. This led to the emergence of the new medical field of tissue engineering1–3 (also called regenerative medical engineering or regenerative tissue engineering). Combining medicine and engineering, tissue engineering forms the basis of regenerative medicine. To regenerate tissues and organs the following three elements need to be placed in a suitable environment: cells that provide the basis for tissue regeneration; a scaffold to support cell differentiation and proliferation; and factors that regulate these processes (Fig. 1). These three elements are known as the triad of tissue engineering. The development of regenerative medicine has been undergirded by recent remarkable developments in fields such as tissue engineering, molecular biology and developmental genetics, and especially by huge advances in stem-cell research, including the generation of induced pluripotent tissue stem cells or progenitor cells. These advances have extended the range of cells that can be used and have found wide application in the development of disease models and the discovery of new drugs. However, clinical applications have been limited as there are many unresolved issues, including medical, ethical and social ones, such as the safety of cell transplantation, sources of tissue stem cells or progenitor cells, and rejection reactions. Whether regenerative medicine will become a major pillar of medical treatment in the future is still uncertain, but some progress in regenerating tissues and organs has already been reported and regenerative medicine is likely to significantly change clinical practice in the near future.
Here, we describe the background and development of the regenerative therapy for the tympanic membrane that we have developed4,5 and present clinical results from a preliminary single-centre study and an investigator-initiated clinical study.
1.1 Structure of the tympanic membrane
The tympanic membrane is a very thin membrane located between the outer and middle ear. It is 8 to 10 mm in diameter and shaped like a funnel, with a concave region known as the umbo in its centre (Fig. 2). The manubrium of the malleus, one of the auditory ossicles, is attached to the tympanic cavity side. A fibrocartilage-like tissue called the tympanic ring connects the outer edge of the tympanic membrane to the external auditory canal bone. The membrane has a trilaminar structure: an epithelial layer, which is continuous with the skin of the external auditory canal; the lamina propria (the middle layer), which consists of fibrous collagen tissue and which imparts strength to the tympanic membrane; and the mucous membrane layer, which is continuous with the mucous membrane of the tympanic cavity. The tympanic membrane consists of two parts: the pars tensa and the pars flaccida. The pars tensa has this trilaminar structure, but the pars flaccida lacks the middle layer, making it susceptible to the impact of pressure in the middle ear. The nutrient vessels that supply the tympanic membrane run in the lamina propria, and their numbers tend to decrease with age. Many nutrient vessels are concentrated around the tympanic ring and the manubrium of the malleus and the umbo in a normal tympanic membrane.
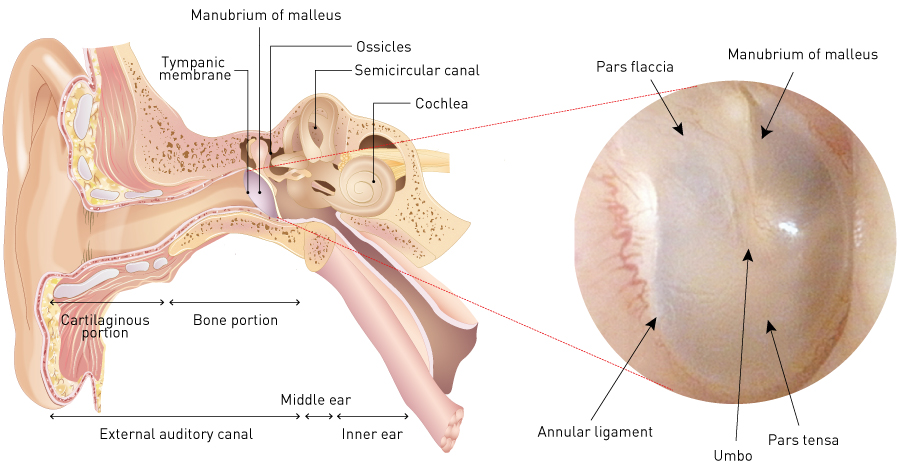
Figure 2. Structure of tympanic membrane.
© [left image: copyright application being submitted]
1.2 Problems caused by perforation of the tympanic membrane
Not only is a perforated tympanic membrane unable to capture sound sufficiently well for normal hearing, but also sound penetrates the tympanic cavity through the perforation, enters the inner ear as vibrational energy through the round window of the cochlea, and ascends the cochlear floor from the bottom (cochlear basal turn) to the top (cochlear apex). Sound entering by the normal route via the residual tympanic membrane and the oval window ascends the scala vestibuli from the bottom to the top through the oval window. When these two vibrations collide with each other near the cochlear apex, energy is rapidly attenuated because of cancellation, resulting in hearing loss. The intelligibility of speech is also reduced, making it difficult to understand spoken words6. Patients with both presbycusis (age-related hearing loss) and tympanic membrane perforations experience significant hearing loss and may require a hearing aid depending on perforation size. A hearing aid further increases the cancellation effect. Consequently, while it amplifies the sound entering the ear, it does not improve word recognition.
When there is a tympanic membrane perforation, the middle ear is directly exposed to the outside via the external auditory canal. The resulting susceptibility to infection can lead to otitis media. Changes in temperature and pressure can damage the inner ear and cause dizziness, which leads to sensorineural hearing loss over time. It is thus desirable to close membrane perforations as much as possible to maintain a good conduction environment in the middle ear and to prevent injury to the inner ear.
1.3 Causes of tympanic membrane perforations
According to the World Health Organization, chronic otitis media affects up to 330 million patients worldwide7. Tympanic membrane perforations usually accompany chronic otitis media, but they can also be caused by trauma, incision, tube placement in the tympanic membrane, burns, radiotherapy and other causes. Thus, the number of patients with tympanic membrane perforations is likely to exceed the above number.
Perforations due to physical or mechanical injury generally heal by themselves within three months in most patients8. Therefore, with the exception of cases where skull fracture or other severe injuries have greatly deformed the tympanic membrane, observation for at least six months is standard first-line management for simple perforations caused by, for example, a cotton bud, a slap in the face, or barotrauma due to rapid pressure changes caused by a blast or air travel. In contrast, persistent perforations following otitis media or tube placement in the tympanic membrane as well as perforations caused by burns or radiotherapy often require some surgical treatment.
1.4 Current treatment of tympanic membrane perforations
The current methods for closing perforations in the tympanic membrane are primarily myringoplasty and tympanoplasty. For small perforations, the surface may be covered with collagen and chitin membranes after transforming the perforation edges into an open wound9. Myringoplasty targets tympanic membrane perforations alone and is performed in patients with a normal ossicular chain and who have no inflammation, infection or lesions in their middle ear. Tympanoplasty involves reconstructing the ossicular chain regardless of the presence of a tympanic membrane perforation and is classified into types I–V based on the severity of defects in the auditory ossicles. Type I tympanoplasty is performed primarily on patients with a normal ossicular chain to suppress inflammation, infection or lesions in the middle ear. In any type of tympanoplasty, autologous tissue such as temporal fascia is collected as material for reconstructing the tympanic membrane, and the epithelial layer of the residual tympanic membrane is detached to insert the autologous tissue between the epithelial layer and the fibrous middle layer. Alternatively, the mucous membrane layer (the deepest layer) around the perforation may be incised to adhere the autologous tissue to the wound site. The tympanic membrane is closed when the upper epithelial layer and the mucous membrane layer are extended with the autologous tissue as the scaffold.
Tympanoplastic methods have high success rates because of the strong affinity between tissues due to the use of autologous tissues for reconstruction. However, tympanic membranes produced by these procedures usually lack a normal middle layer because it is harder to regenerate that fibrous layer than the other two layers in the tympanic membrane. The transplanted autologous tissue may gradually degenerate, resulting in a weak tympanic membrane that lacks a middle layer. Alternatively, if it does not degenerate, a very thick tympanic membrane may result. A mixture of these two patterns may occur. New perforations at this site rarely close spontaneously.
If the ossicular chain is normal, hearing recovers to some extent after closing the perforation, but perfect hearing with no air–bone gap is hard to achieve because the conduction efficiency varies depending on both the degree of contact with the malleus and the thickness of the tympanic membrane. Incision of the postauricular skin and collection of autologous tissue for reconstructing the tympanic membrane may result in various sequelae, such as sensory loss and discomfort around the auricle.
2. THE BIOLOGY AND NON-CLINICAL PROOFS OF CONCEPT OF NEW TREATMENTS
2.1 Healing mechanism for tympanic membrane perforations
Since small perforations in healthy tympanic membranes caused by trauma often heal, many studies have focused on this mechanism for regenerating the membrane10–13. Most of these studies are animal experiments, and they have shown that the epithelial layer in the trilaminar structure of the tympanic membrane extends first and that the two other layers then extend using the epithelial layer as the scaffold10. Thus, each layer has a different rate of regeneration and a different capacity to regenerate. The fibrous middle layer gives strength and elasticity to the tympanic membrane, but it regenerates slower than the epithelial layer above it and the mucous membrane layer beneath it. Therefore, if the epithelial and mucous membrane layers, which regenerate faster, adhere to each other in regions beyond the middle layer, the fibrous layer will lack space to grow further and the perforation will persist if regeneration of the epithelial and mucous membrane layers stops at this point. If regeneration proceeds and the perforation is closed, a fragile and transparent tympanic membrane will form that lacks a fibrous layer (Fig. 3). The edge of the regenerated fibrous layer turns white. A slight pressure change can then cause reperforation.
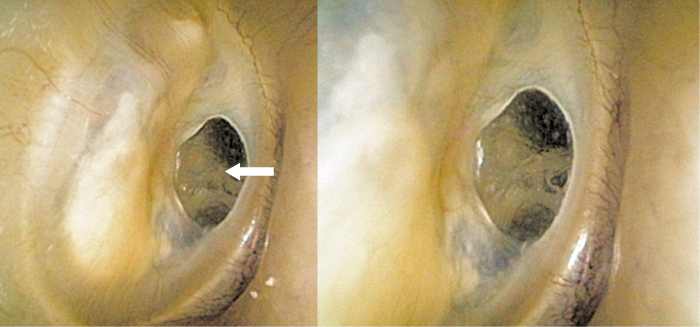
Figure 3. Tympanic membrane that has healed spontaneously. The white arrow points to a transparent area of tympanic membrane that has formed without the middle layer. No nutrient vessels can be seen. The edge of the middle layer can be observed as a white margin surrounding the transparent tympanic membrane.
The tympanic membrane’s regenerative capacity indicates the presence of tissue stem cells or progenitor cells in the membrane and its surroundings. Indeed, animal experiments have shown that these cells probably exist in the tympanic membrane ring and the malleus (umbo and manubrium) and that injuring the membrane initiates their active proliferation14,15. Normally, these cells only support cell turnover in the tympanic membrane, but an injury to the membrane appears to trigger the repair system and initiate active reactions. However, these cells are dormant in chronic tympanic membrane perforations. Thus, when the tympanic membrane is injured, the regeneration mechanism is switched on by the injury itself. However, if the regeneration environment is unfavourable (as is the case in an infection and chronic inflammation) or if the perforation is large, cell growth stops prematurely and the perforation persists. It is thus critical to maintain a favourable regeneration environment.
Observation of the natural course of tympanic membrane regeneration indicates that it is necessary to:
- promote growth of the fibrous middle layer of the membrane;
- provide a scaffold on which each membrane layer can grow;
- maintain a suitable regeneration environment that efficiently promotes membrane regeneration.
2.2 Promoting growth of the fibrous middle layer of the tympanic membrane
Various growth factors have been found for the fibrous middle layer of the tympanic membrane, but few are suitable for clinical application. Among those that are suitable, basic fibroblast growth factor (bFGF) is already marketed as a drug product in Japan, and its use for treating skin ulcers is covered by health insurance. bFGF directly affects cell proliferation and blood vessel growth16,17.
During regeneration of a normal tympanic membrane, it is essential to maintain enough blood flow to support regeneration of the trilaminar structure, particularly the fibrous middle layer, which regenerates very slowly. bFGF is therefore ideal as it directly affects the growth of fibroblasts essential for regenerating the fibrous layer18 and also induces blood vessels to supply nutrients to the surrounding tissues.
2.3 Providing a scaffold on which each tympanic membrane layer can grow
In tissue regeneration, selection of a scaffold for cell growth is critical. A scaffold needs to have a high tissue affinity and be able to be eliminated by absorption or degradation after serving as a scaffold.
Our tympanic membrane regeneration therapy involves impregnating a bioabsorbable scaffold material with the growth factor, placing it within the tympanic membrane defect and fixing it with a bioadhesive glue. We selected gelatin sponge as the scaffold material for regenerating the tympanic membrane. Its primary component is the triple-helical structure of collagen molecules uncoiled by heat denaturation. When a liquid is added, it becomes a free-shaped pliable mass and has a much looser structure than collagen (Fig. 4). Gelatin sponge is hydrolyzed and eliminated from the body within 1 month19,20, although it may take a few months to eliminate it from the tympanic membrane because the tympanic cavity contains air and is not in living tissue.
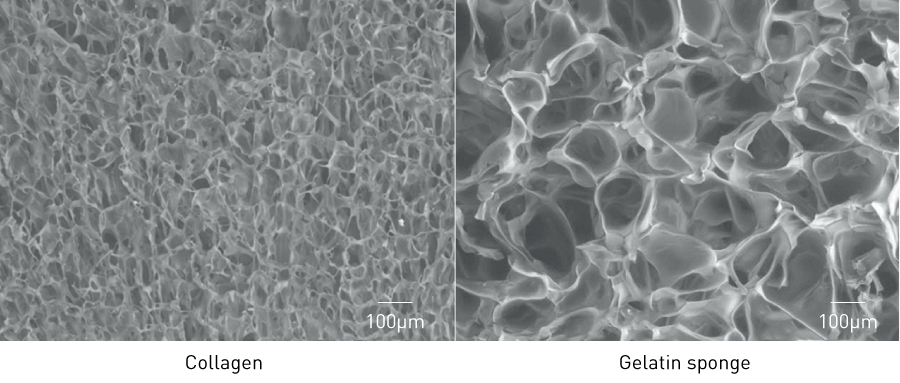
Figure 4. Difference in density between collagen and gelatin sponge (electron micrographs).
Our therapy differs from many conventional ones in that it uses free-shaped gelatin sponge as a scaffold. Conventional therapies repair defects in the tympanic membrane by extending the surrounding tissues along the inner and outer surfaces of the sheet-like scaffold material glued to the portion of the tympanic membrane with the defect. If the membrane perforation is small and the entire defective portion is flat, it can be effectively repaired by conventional methods. If the defect is larger however, it is difficult to cover the entire defective portion with a flat sheet of material because of the complicated shape of the tympanic membrane and the auditory ossicle attached to it. If the defective portion cannot be completely covered, the tympanic membrane cannot be repaired without a scaffold, or the membrane may fuse with the surrounding tissue, resulting in sequelae. In fact, if the tympanic membrane defect exceeds a certain size or if part of the auditory ossicle is affected, conventional therapies will fail to repair the perforation because the scaffold cannot cover the entire defect.
In contrast, gelatin sponge can easily cover an entire defect regardless how complex its shape is. And it is effective even for defects that span the entire tympanic membrane. This scaffold material has enabled the successful regeneration of a tympanic membrane with large perforations and virtually no residual membrane21. Gelatin sponge can also release bFGF in a sustained manner22, which is advantageous for tympanic membrane regeneration.
Observation of a patient whose tympanic membrane had been completely regenerated by this therapy (Fig. 5) reveals that part of the gelatin sponge remains inside the tympanic membrane (in the tympanic cavity), and the gelatin sponge on the outside (in the external auditory canal) has separated as a crust. This indicates that the regenerated tympanic membrane grows through the inside of the very loose open structure of the gelatin sponge (Fig. 4), which shows that the therapy is based on a different concept from that of conventional tympanic membrane regeneration. Tissues do not regenerate through the inside of a dense scaffold, such as the sheet-like collagen membrane used in conventional therapy, but rather grow along the surface of the scaffold. But because a loosely structured scaffold such as gelatin sponge does not interfere with the expansion of cells, regenerative cells can pass through the inside of the loose structure to repair the tympanic membrane.
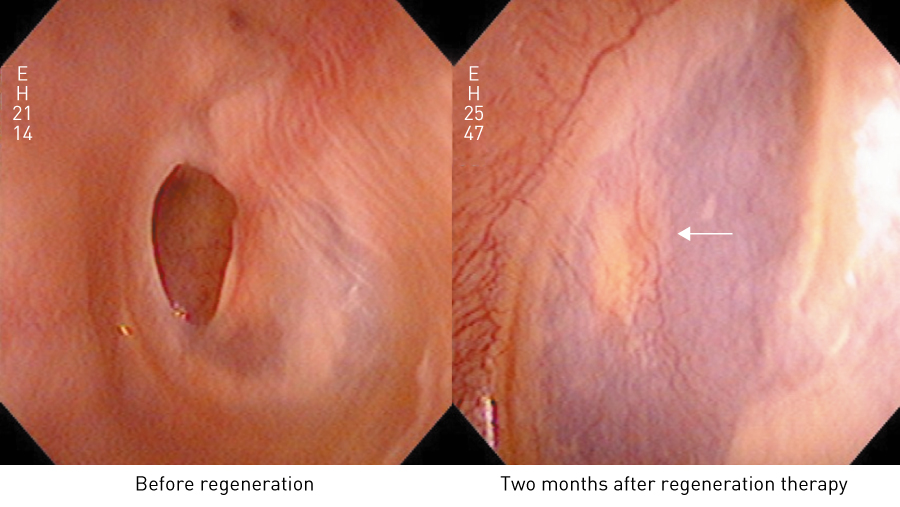
Figure 5. Observation of regenerated tympanic membrane. A regenerated tympanic membrane with the appearance of frosted glass and numerous capillaries two months after tympanic membrane regeneration therapy, indicating that the middle layer has regenerated. The white arrow points to the remaining gelatin sponge.
2.4 Maintaining a favourable regeneration environment
For tissue regeneration to occur, the conditions must be suitable for in vivo cell culture. In our therapy, the scaffold is fixed in place for a certain time by dripping fibrin glue onto it and thereby covering the surface of the gelatin sponge with a fibrin membrane. The inside of the gelatin sponge is kept moist to isolate the culture environment from the outside.
3. TREATMENT PROCEDURE
Tympanic membrane regenerative therapy is based on the concept of in situ tissue engineering. The tissue engineering elements used are endogenous tissue stem cells or progenitor cells, gelatin sponge (Spongel®, Astellas Pharma Inc., Tokyo, Japan) as a scaffold and basic bFGF (Fiblast®, Kaken Pharma Co., Ltd, Tokyo, Japan) as a regulatory factor. A fibrin glue (Bolheal®, Chemo-Sero-Therapeutic Research Institute [Kaketsuken], Kumamoto, Japan; Beriplast®, CSL Behring K.K., Tokyo, Japan) is also used to prepare a favourable regeneration environment. Cells are not transplanted in this environment; rather the edge of the tympanic membrane perforation is made into an open wound, which activates dormant endogenous tissue stem cells or progenitor cells.
Figure 6 shows the steps involved in the therapy. To anaesthetize the external auditory canal, including the residual tympanic membrane around the perforation and its surroundings, a swab impregnated with 4% lidocaine is inserted and kept in place for 15–20 minutes. The edge of the tympanic membrane perforation is then transformed into an open wound using a myringotomy knife or a Rosen probe. At this point, the circumferential edges of all three layers of the tympanic membrane are exposed.
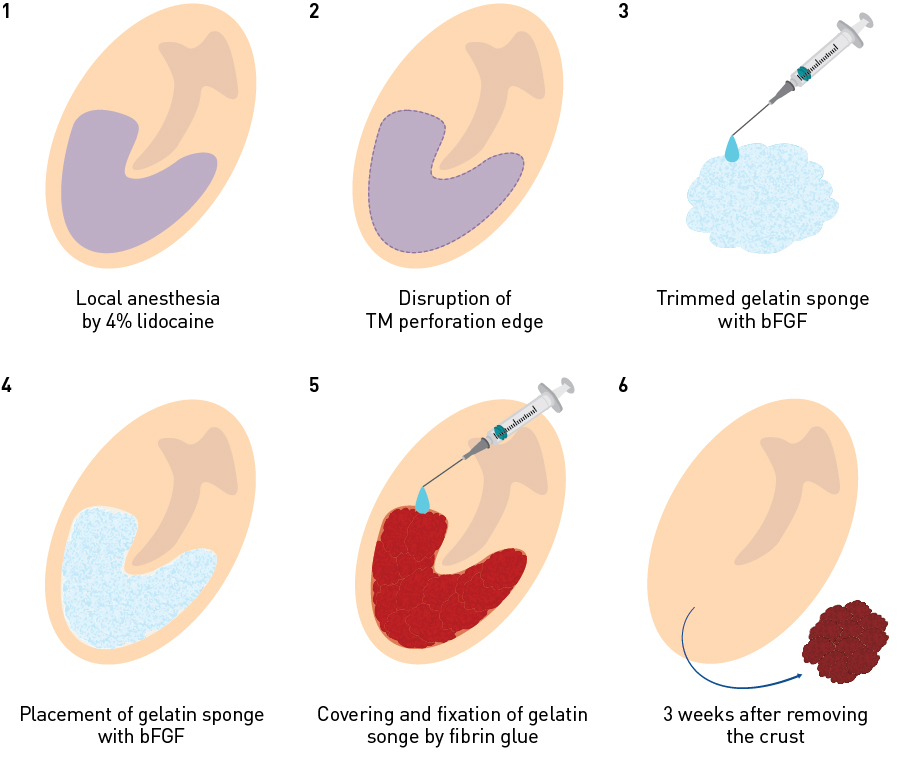
Figure 6. Steps involved in tympanic membrane regeneration therapy. 1. Infiltration anesthesia of the remaining tympanic membrane and the tympanic membrane perforation edge with a 4% lidocaine swab; 2. Transformation of the tympanic membrane edge into an open wound; 3, 4. Placement of bFGF-impregnated gelatin sponge; 5. Covering the bFGF-impregnated gelatin sponge with fibrin glue; 6. Removal of the crust three weeks after treatment.
Gelatin sponge impregnated with bFGF is then trimmed to fit the size of the tympanic membrane perforation and placed between the tympanic cavity and the external auditory canal. It is important to place sufficient sponge pieces to sandwich the residual tympanic membrane from both the tympanic cavity side and the external auditory canal side, so that the gelatin sponge is in complete contact with the whole tympanic membrane perforation edge and there are no gaps. Finally, a few drops of a fibrin glue are dripped from a syringe to cover and stick to the entire gelatin sponge. After three weeks, the wound crust is removed from the upper surface of the tympanic membrane to check for regeneration. If the crust adheres to the surface, it may be removed by inserting a swab impregnated with 4% lidocaine from the external auditory canal for 15–20 minutes. If complete regeneration does not occur after the first treatment, the treatment may be repeated after removing the gelatin sponge retained in the tympanic cavity or the external auditory canal. Retreatment should be limited to four attempts, as clinical experience has shown that the probability of regenerating the tympanic membrane is very low beyond the fourth treatment. If otorrhea due to infection or inflammation is observed after regeneration treatment, further treatments are suspended, and oral or eardrop antibiotic therapy is initiated to suppress inflammation and otorrhea. Treatment is resumed after adequate healing (which may be several months). There may be allergic reactions to gelatin sponge, bFGF, or fibrin glue in rare cases.
4. CLINICAL RESULTS
4.1 Preliminary single-centre study
4.1.1 Selection of patients and materials
This new therapy was approved by the institutional review board of Kanai Hospital in Kyoto. And patients were limited to those who fully understood the procedure and agreed to pursue this new treatment by signing informed consent documents. To examine the efficacy and safety of tympanic membrane regenerative therapy in a clinical setting, we first conducted a single-centre preliminary study. This involved 218 patients (232 ears) with tympanic membrane perforations and no confirmed active infection or inflammation in the middle or outer ear. The causes of tympanic membrane perforations were chronic otitis media (N = 136), old traumatic tympanic membrane perforations (N = 42), persistent perforations after tympanic membrane incision or tympanic membrane tube placement due to exudative otitis media (N = 26), reperforations after closure of tympanic membrane perforations or tympanoplasty (N = 15), burns (N = 4), post-irradiation (N = 3) and unknown (N = 6). The male/ female ratio was 101:117. Patients ranged from 8 to 91 years of age.
Patients were divided into three groups according to perforation size. Perforations occupying less than 1/3, 1/3 to 2/3 and more than 2/3 of the tympanic membrane were classified as grades I, II and III, respectively.
4.1.2 Treatment method
The treatment procedures are described above. After treatment, patients were instructed not to apply pressure to the ear by strong sniffing or nose blowing, to use caution when washing their hair or having a bath so that water would not enter the affected ear and to visit the outpatient clinic 3 weeks after surgery.
4.1.3 Assessment
The success of perforation closure was assessed according to the size of the perforation, the number of treatments required for closure and the degree of hearing improvement three months after the final treatment. Occurrences of adverse events were recorded.
4.1.4 Results
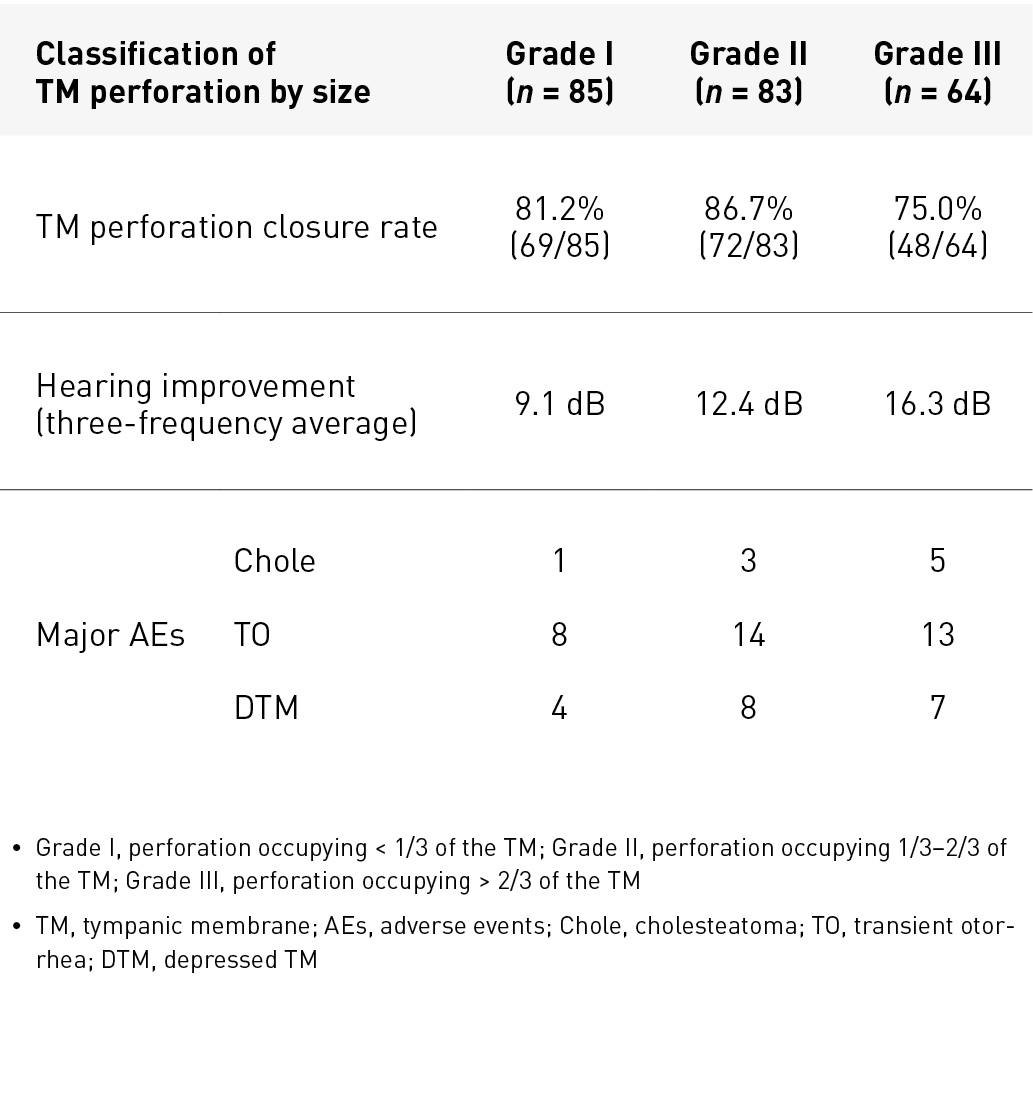
Table 1. Results of preliminary single-centre study of tympanic membrane regeneration therapy
Table 1 shows the results, while Fig. 7 shows an example of regeneration. Overall, the tympanic membrane perforation closure rate (189/232 ears) was 81%, and favourable hearing improvement was achieved with a small air–bone gap. Of the 189 ears with successful tympanic membrane regeneration, 80% or more had perforation closure with two or fewer treatments (Fig. 8). The most common adverse event was transient otorrhea (35/232 ears, 15.1%). Some cases of depressed tympanic membrane (19/232 ears, 8.2%) required tympanic membrane incision (5/232 ears, 2.2%). Additionally, cholesteatoma (9/232 ears, 3.4%) occurred under the epithelium of the tympanic membrane, but all lesions could be removed with a probe.
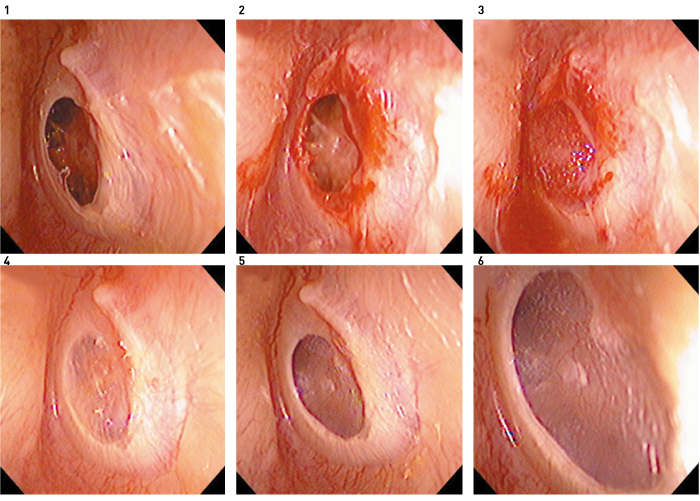
Figure 7. Tympanic membrane regeneration in a 65-year-old woman who had chronic otitis media for 30 years. 1, Before regeneration treatment; 2, transformation of the tympanic membrane perforation edge into an open wound; 3, placement of bFGF impregnated gelatin sponge and covering with fibrin glue; 4, regenerated tympanic membrane and remaining gelatin sponge in the tympanic cavity 3 weeks after treatment; 5,6, the gelatin sponge in the tympanic cavity is eliminated, and numerous capillaries are observed in the regenerated tympanic membrane 4 months after treatment.
Reprinted from Ref. 4, with permission
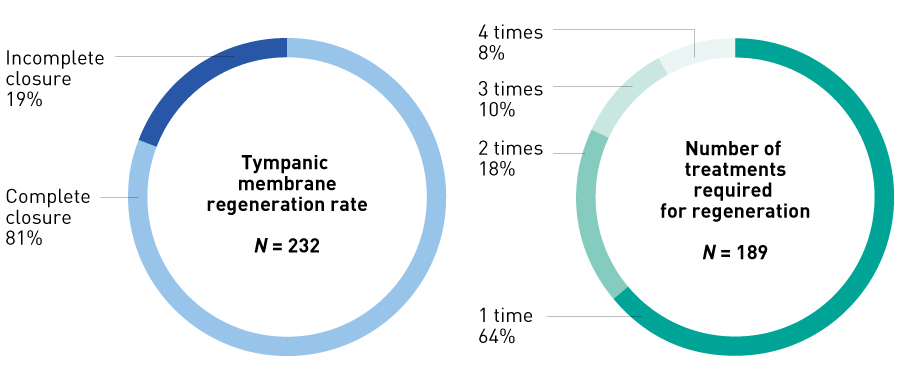
Figure 8. Tympanic membrane regeneration rate and number of treatments required for regeneration in preliminary study. The tympanic membrane regeneration rate after a maximum of four treatments was 81% (189/232 ears), and 82% of tympanic membrane regenerations were achieved with two or fewer treatments.
Regeneration is difficult if the tympanic membrane ring or the malleus has been lost during tympanoplasty or by other causes. Regeneration was successful in some patients with the tympanic membrane ring and the malleus conserved in tympanoplasty, and the regeneration rate (4/15 ears) was only 26.7%. No regeneration occurred in membrane perforations caused by burns or radiotherapy. These results suggest that tissue stem cells or progenitor cells may have been eliminated in these patients. Reperforation occurred in four patients (1.3%, 4/232 ears) during the three-month observation period, and all these perforations were about the size of a pin hole.
5. INDICATIONS FOR TYMPANIC MEMBRANE REGENERATION
5.1 Exclusion criteria for tympanic membrane regeneration
Only 20–30% of patients with perforations meet the eligibility criteria for tympanic membrane regeneration. Finding ways to increase the number of eligible patients in the future is thus important. The exclusion criteria for receiving tympanic membrane regenerative therapy shown below are based on the results of previous clinical studies:
- The patient has active infection or inflammation in the tympanic cavity or the external auditory canal as well as otorrhea.
- The tympanic membrane, tympanic cavity and external auditory canal are not dry, or advanced calcification is observed in the residual tympanic membrane.
- High-resolution computed tomography of the temporal bone shows a soft tissue shadow in the tympanic cavity or the mastoid cavity.
- The patient has a history of middle-ear surgery such as tympanoplasty or myringoplasty.
- The tympanic membrane perforation was caused by radiation or burns.
- The normal structure of the middle ear is lost (including loss of the tympanic membrane ring and fusion of the residual tympanic membrane).
- The edge of the perforation cannot be directly observed using microscopy because of a narrow external auditory canal.
These criteria can be divided into three categories. Criteria 1, 2 and 3 represent a poor regeneration environment; criteria 4, 5 and 6 represent a partial or total lack of tissue stem cells or progenitor cells supporting tympanic membrane regeneration; and criterion 7 represents a condition where the entire circumferential edge of the perforation cannot be transformed into an open wound because of the narrow external auditory canal.
5.2 Expansion of indications
Among the exclusion criteria, a lack of tissue stem cells or progenitor cells presents the most difficulties for regeneration, and co-transplantation of cells with the scaffold is the only conceivable regeneration method. For criteria 1, 2 and 3, tympanic membrane regeneration can be performed if the regeneration environment is improved. For criterion 7, where the perforation edge cannot be directly observed by microscopy because of a narrow external auditory canal, tympanic membrane regeneration can be performed endoscopically if an endoscope can be used to observe the entire tympanic membrane and perform treatment. If observation and treatment are still difficult, tympanic membrane regeneration may be performed after expansion of the external auditory canal. All these solutions are more straightforward than those for criteria 4, 5 and 6. Where a soft tissue shadow is present in the tympanic or mastoid cavity (criterion 3), such lesions usually need to be removed by opening and drilling the bone using a procedure such as tympanomastoidectomy. In conditions corresponding to criterion 6, a significant loss of middle-ear structure and large displacement of the residual tympanic membrane due to adhesive otitis media make regeneration very difficult.
5.3 Improving the regeneration environment before regeneration
If the inside of the ear is wet during the inactive phase of chronic otitis media or the patient has active infection or inflammation and otorrhea that is considered to be acute exacerbation of chronic otitis media, but meets other eligibility criteria, conservative treatment is first performed with oral antibiotics and eardrops, and followed up for one to two months. When inflammation has been suppressed, the tympanic cavity is washed repeatedly in the operating room, mucus in the tympanic cavity is completely removed with a swab, and the patient continues with tympanic membrane regenerative therapy. This method resulted in a tympanic membrane regeneration rate of 70–80% in our patients (Fig. 9)21.
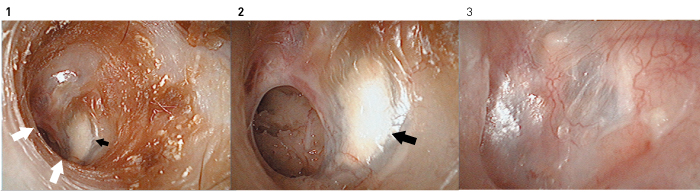
Figure 9. Tympanic membrane regeneration in a 39-year-old woman with chronic otitis media. Fibrescope images (1, 2) before treatment and (3) two months after treatment. The patient had otorrhea and calcification in the remaining tympanic membrane (black arrows). The perforation edge could not be directly observed microscopically because of the extension of the anterior and lower walls of the external auditory canal (white arrows). Regeneration treatment was performed after washing the inside of the tympanic cavity and removing the calcified remaining tympanic membrane endoscopically. The regenerated tympanic membrane is virtually normal without calcification 2 months after treatment.
For patients with advanced calcification or ectopic ossification in the residual tympanic membrane or epithelial invasion (cholesteatoma) into the back of the tympanic membrane, all the residual tympanic membrane is removed before regeneration (Fig. 9).
For patients with a lesion in the tympanic cavity, mainly cholesteatoma or tumour confined to the tympanic cavity, the cholesteatoma or tumour is removed before starting membrane regeneration. The lesion may be removed microscopically or endoscopically, but the inside of the tympanic cavity must be observed endoscopically after removal to check whether there is any remaining lesion. Among cases of cholesteatoma confined to the tympanic cavity, congenital cholesteatoma is most suitable for regeneration treatment. Among tumours, glomus tympanicum tumours are suitable. The membrane regeneration rate is very high among patients with these conditions, being 95% or higher in our patients21.
5.4 Expansion of the external auditory canal
Many patients have a narrow external auditory canal. The external auditory canal has a tubular structure 30–35 mm long and 10 mm in diameter. The proximal half from the entrance of the external auditory canal is the cartilaginous portion, and the distal half is the bone portion. Although there are some differences in shape between adults and children, the section connecting the cartilaginous portion and the bone portion has a slight bend (Fig. 2). In many patients with a narrow external auditory canal, the edge of the perforation cannot be directly observed microscopically because of an extension of the bone portion. The perforation edge can be transformed into an open wound by using an endoscope in most patients. However, because surgical instruments cannot be inserted together with an endoscope into an ear with a very long extension of the bone portion, the external auditory canal needs to be expanded.
To expand the external auditory canal, incisions are made on the luminal surface of the narrow lesion of the external auditory canal longitudinally and perpendicularly to it, in order to peel back part of the soft tissue on the surface and expose the surface of the extended bone. This is removed with a diamond bur, and the surface is smoothed. The external auditory canal is expanded until the edge of the perforation can be sufficiently well observed for regeneration treatment. The detached soft tissue of the external auditory canal is replaced, and bFGF-impregnated gelatin sponge pieces used for tympanic membrane regeneration are placed around the exposed bone and fixed with fibrin glue. If the bone is exposed over a wide area, gelatin sponge is used to fill the external auditory canal and fixed with fibrin glue. The entire auricle is covered and sealed with medical film. The filled gelatin sponge is removed after three weeks. The soft tissue of the external auditory canal and the tympanic membrane can both be regenerated simultaneously using this method22,23.
6. FUTURE PROSPECTS
6.1 Prospects and issues for chronic otitis media
The primary goal of tympanic membrane regeneration is to restore ideal hearing with no air–bone gap by minimally invasive surgery. There are several advantages of closing the tympanic membrane, including improved hearing, relief of discomfort, prevention of otitis media and, in the longer term, protection of the middle and inner ear. However, hearing is not only affected by tympanic membrane perforations, but also by complex factors relating to other structures in the middle and inner ear and to the retrocochlear region and the central nervous system. For example, if tympanic membrane regeneration is performed in patients with a perforation and a poorly functioning eustachian tube due to poor growth of mastoid air cells, depression of the tympanic membrane and exudative otitis media may occur, because the middle ear pressure, which was regulated via the perforation, is no longer regulated, and hearing recovery may be inadequate.
As tympanic membrane perforations are mainly caused by chronic otitis media, eligibility for membrane regeneration is most critical for patients with this condition. Many patients with chronic otitis media have lesions in the mastoid cavity as well as in the tympanic cavity. A combination of tympanic membrane regeneration and conventional tympanomastoidectomy is recommended for patients with lesions in both cavities. To establish adequate communication between the tympanic and mastoid cavities, the lesions in both cavities are removed by procedures such as tympanomastoidectomy. Membrane regenerative therapy, rather than the usual autologous tissue transplantation, is performed to reconstruct the tympanic membrane. However, to allow for tympanic membrane regeneration, it is essential not to injure the tympanic membrane ring when detaching all tympanic membrane layers in order to retain tissue stem or progenitor cells and to retain the residual tympanic membrane attached to the malleus, and the umbo and manubrium15,16. This therapy is suitable for only certain patients, and it requires a high level of surgical skill. In addition, otitis media with cholesteatoma will reoccur if the mechanisms are not improved that reduce pressure in the middle ear, since impaired middle ear gas exchange leads to depression of the upper tympanic cavity and the formation of cholesteatoma. Because adhesive otitis media is also caused by reduced pressure in the middle ear, a simple combination of tympanomastoidectomy and tympanic membrane regeneration cannot cure the disease completely. Poor mastoid cell growth leads to a poorly functioning eustachian tube. They facilitate middle ear gas exchange in a complementary way. Therefore, regeneration of mastoid air cells may be needed in combination with these procedures to improve middle ear gas exchange23,24.
6.2 Prospects and issues for patients lacking tissue stem cells or progenitor cells
Regeneration is most difficult when tissue stem cells or progenitor cells are not present. Obtaining tissue stem cells or progenitor cells is hence a vital issue. We previously reported that these cells are present in the tympanic membrane ring and the malleus (umbo and manubrium)15,16. Both the tympanic membrane ring and the malleus are essential to regenerate the tympanic membrane with a complex shape. One method uses the tympanic membrane ring and tympanic membrane, including the malleus, from a cadaver as a scaffold. It involves decellularizing, removing antigens, culturing autologous mesenchymal tissue stem cells or progenitor cells and transplanting these cells together with the scaffold. In this method, the question of whether to perform partial or full transplantation needs to be considered for each patient, but the method can be used in combination with tympanoplasty because the tympanic membrane and the auditory ossicle do not vary greatly in size between individuals.
6.3 Prospects for tympanic membrane regeneration
Otologic surgeries, such as common myringoplasty, require a fully equipped operating room and a wide variety of surgical instruments and are usually performed under general anesthesia. In addition to incision of the postauricular skin and collection of autologous tissues such as temporal fascia, these surgeries require multistep surgical procedures such as detaching the external auditory canal, opening and drilling the bone, raising the epithelial layer of the tympanic membrane, transforming the perforation edge into an open wound, transplanting the temporal fascia and tamponing. At least 1 hour of surgery is required. In addition, patients need to rest in bed and limit daily activities to prevent infection and displacement of the transplanted fascia. An even bigger challenge is training surgeons well enough to perform these otologic surgeries, because training takes a long time and requires the input of senior surgeons. In contrast, tympanic membrane regenerative therapy for a simple perforation involves only transforming the perforation edge into an open wound and placing the gelatin sponge. Treatment takes only about 15 minutes. Treatment can be performed on a chair or a cot bed in an outpatient setting, and only a few types of instrument are needed, including a simple microscope or endoscope. Hospitalization or bed rest is unnecessary after surgery, and patients need to visit the clinic for a checkup only once every three or four weeks after treatment. Physicians can be quickly trained in this therapy because the treatment is very simple and non-invasive compared with conventional surgical procedures. This therapy costs only a fraction of tympanic membrane regenerative therapy combined with myringoplasty. As well as being cost effective, tympanic membrane regenerative therapy realizes closure of the tympanic membrane perforation for a similar percentage of patients as conventional surgeries, while it achieves far better hearing.
Convenience and low cost are great advantages in developing countries, where the therapy is needed most by young patients. In developed countries, the therapy will lower the psychological hurdle for middle-aged and senior patients who have given up on treatment for tympanic membrane perforations and, in addition to improving hearing, it will reduce the risks of dementia due to hearing loss.
References
- Langer, R. & Vacanti, J. P. Tissue engineering. Science 260, 920–926 (1993).| article
- Vacanti, J. P., Morse, M. A., Saltzman, W. M., Domb, A. J., Perez-Atayde, A. et al. Selective cell transplantation using bioabsorbable artificial polymers as matrices. J. Pediatr. Surg. 23, 3–9 (1988). | article
- Vacanti, C. A., Kim, W., Upton, J., Vacanti, M. P., Mooney, D. et al. Tissue-engineered growth of bone and cartilage. Transplant. Proc. 25, 1019–1021 (1993). | article
- Kanemaru, S., Umeda, H., Kitani, Y., Nakamura, T., Hirano, S. et al. Regenerative treatment for tympanic membrane perforation. Otol. Neurotol. 32, 1218–1223 (2011). | article
- Omae, K., Kanemaru, S., Nakatani, E., Kaneda, H., Nishimura, T. et al. Regenerative treatment for tympanic membrane perforation using gelatin sponge with basic fibroblast growth factor. Auris Nasus Larynx 44, 664–671 (2017). | article
- Ahmad, S. W. & Ramani, G. V. Hearing loss in perforations of the tympanic membrane. J. Laryol. Otol. 93, 1091–1098 (1979). | article
- Acuin, J. Chronic suppurative otitis media : burden of illness and management options. World Health Organization (Geneva, 2004, WHO). | article
- Jin, Z.-H., Lou, Z.-H. & Lou, Z.-C. Assessment and spontaneous healing outcomes of traumatic eardrum perforation with bleeding. Am. J. Otolaryngol. 38, 479–483 (2017). | article
- Kim, J. H., Choi, S. J., Park, J.-S., Lim, K. T., Choung, P. H. et al. Tympanic membrane regeneration using a water-soluble chitosan patch. Tissue Eng. A. 16, 225–232 (2010). | article
- Johnson, A. P., Smallman, L. A. & Kent, S. E. The mechanism of healing of tympanic membrane perforations. Acta Oto-Laryngol. 109, 406–415 (1990). | article
- Fina, M., Bresnick, S., Baird, A. & Ryan, A. Improved healing of tympanic membrane perforations with basic fibroblast growth factor. Growth Factors 5, 265–272 (1991). | article
- Mondain, M. & Ryan, A. Histological study of the healing of traumatic tympanic membrane perforation after basic fibroblast growth factor application. Laryngoscope 103, 312–318 (1993). | article
- Ma, Y., Zhao, H. & Zhou, X. Topical treatment with growth factors for tympanic membrane perforations: progress towards clinical application. Acta Oto-Laryngol. 122, 586–599 (2009). | article
- Knutsson, J., von Unge, M, & Rask-Andersen, H. Localization of progenitor/stem cells in the human tympanic membrane. Audiol. Neurootol. 16, 263–269 (2011). | article
- Liew, L. J., Chen, L. Q., Wang, A. Y., von Unge, M., Atlas, M. D. et al. Tympanic membrane derived stem cell-like cultures for tissue regeneration. Stem Cells Dev. 27, 649–657 (2018). | article
- Virag, J. A. I., Rolle, M. L., Reece, J., Hardouin, S., Feigl, E. O. et al. Fibroblast growth factor-2 regulates myocardial infarct repair: effects on cell proliferation, scar contraction, and ventricular function. Am. J. Pathol. 171, 1431–1440 (2007). | article
- Vrabec, J. T., Schwaber, M. K., Davidson, J. M. & Clymer, M. A. Evaluation of basic fibroblast growth factor in tympanic membrane repair. Laryngoscope 104, 1059–1064 (1994). | article
- Kato, M. & Jackler, R. K. Repair of chronic tympanic membrane perforations with fibroblast growth factor. Otolaryngol. Head Neck Surg. 115, 538–547 (1996). | article
- Rohanizadeh, R., Swain, M. V. & Mason, R. S. Gelatin sponges (Gelfoam) as a scaffold for osteoblasts. J. Mater. Sci. Mater. Med. 19, 1173–1182 (2008). | article
- Komura, M., Komura, H., Kanamori, Y., Tanaka, Y., Suzuki, K. et al. An animal model study for tissue-engineered trachea fabricated from a biodegradable scaffold using chondrocytes to augment repair of tracheal stenosis. J. Pediatr. Surg. 43, 2141–2146 (2008). | article
- Kanemaru, S. I., Kanai, R., Yoshida, M., Kitada, Y., Omae, K., & Hirano, S. Application of regenerative treatment for tympanic membrane perforation with cholesteatoma, tumor, or severe calcification. Otol. Neurotol. 39, 438–444 (2018). | article
- Takemoto, S., Morimoto, N., Kimura, Y., Taira, T., Kitagawa, T., Tomihata, K. et al. Preparation of collagen/gelatin sponge scaffold for sustained release of bFGF. Tissue Eng. A 14, 1629–1638 (2008). | article
- Kanemaru, S., Umeda, H., Kanai, R., Tsuji, T., Kuboshima, F. et al. Regenerative treatment for soft tissue defects of the external auditory meatus. Otol. Neurotol. 35, 442–448 (2014). | article
- Kanemaru, S., Nakamura, T., Omori, K., Magrufov, A., Yamashita, M. et al. Regeneration of mastoid air cells in clinical applications by in situ tissue engineering. Laryngoscope 115, 253–258 (2005). | article