Quick links to specific parts of this article:
- Researching and developing novel medical technologies
- Promoting, managing, and supporting the operation of clinical trials and large-scale cohort studies
- Disseminating medical and clinical research information
Researching and developing novel medical technologies
Through multiple projects commissioned by the Japanese government, TRI has worked towards establishing a foundation that will support and promote Japan’s translational research and will lead to:
- Strengthened academic R&D pipelines
- More networks between academic research organizations
TRI supports institutions by communicating with corporate bodies and governments, in order to yield practical applications from the ‘seeds’ created by each TR center to overcome intractable diseases.
TRI has also shared their translational research expertise with the world, through programs including:
- The Cancer TR Program
- The Coordination, Support and Training Program for TR
- The TR Network Program
- The TR Center Workshop
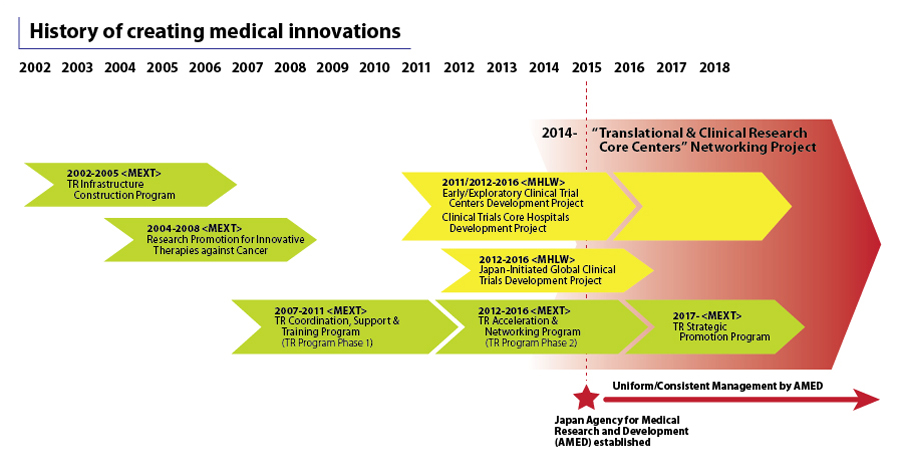
TRI, in collaboration with the Japan Agency for Medical Research and Development (AMED), has supported the Translational & Clinical Research Core Centers Networking Project — a programme that aims to establish academic R&D pipelines in Japan to facilitate innovations in drugs, medical devices and technology — in creating a total of 1,000 pipelines.
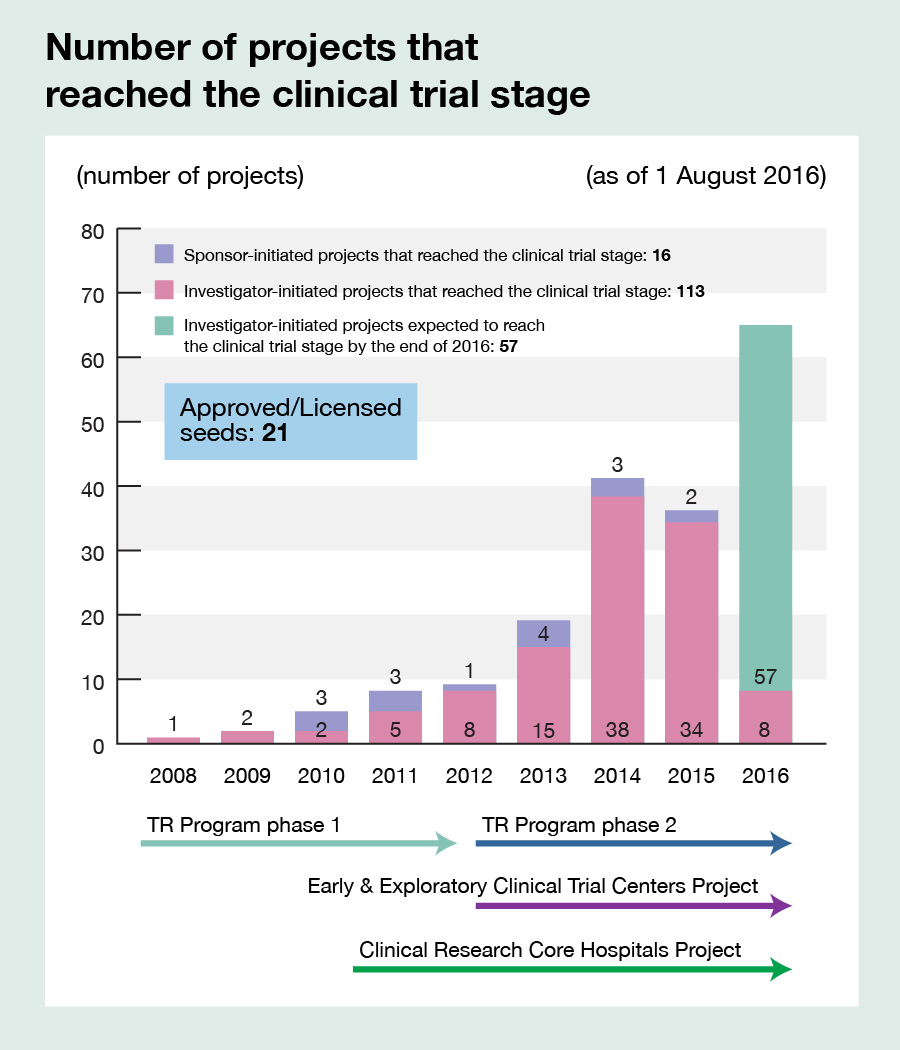
Promoting, managing, and supporting the operation of clinical trials and large-scale cohort studies
TRI offers a range of research consultation services on:
Development strategies
- To help develop policies (for example, on marketing research, regulatory classification and development schemes)
- Patent strategies (including consultation on patents and research for patent applications)
- Collaborations with joint developers
- Organizational support for academic research organizations
Clinical studies
- Protocol development
- Study management
- Data management
- Statistical analyses
- Electronic Data Capture system development
- Support for global clinical trials regarding planning, launch and operation
- Monitoring
- Audit
For further information about our research consultation services, please click here
or email: 
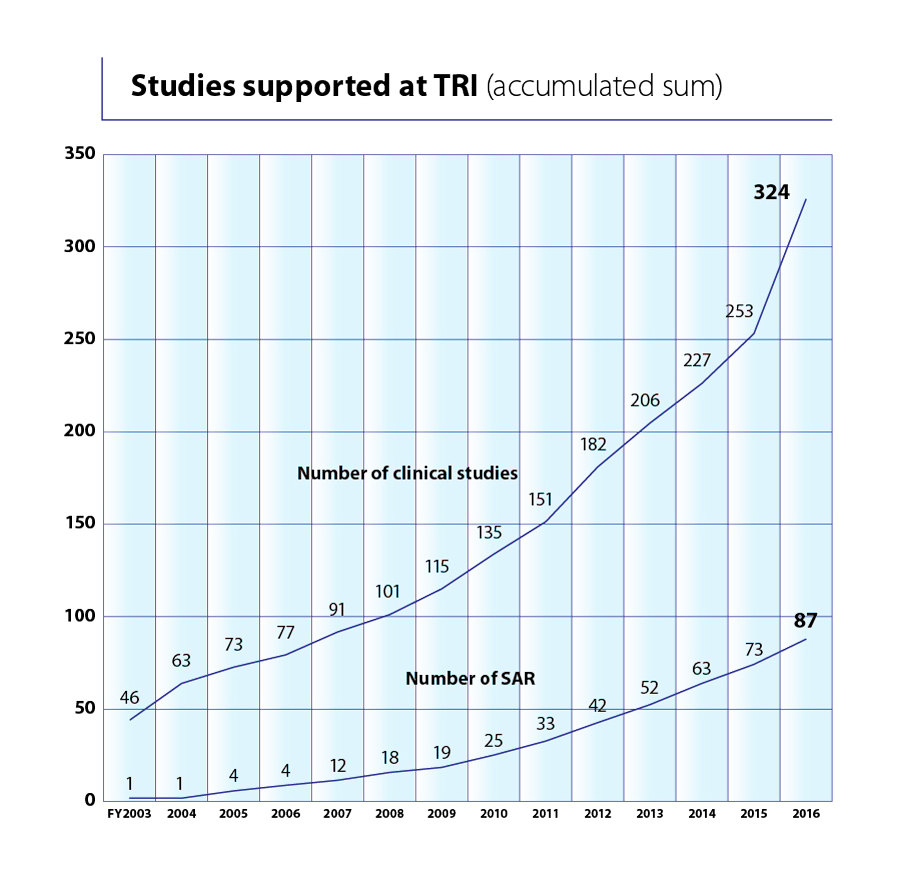
TRI also offers comprehensive support for clinical studies – all the way from planning through to publication. This support includes:
Planning
- R&D consultation
- Support for protocol drafts
- Support for protocol development
- Preparation of patient registration forms and case report forms
- Support for preparation of informed consent forms
- Preparation for adverse event handling manuals
- Development of Electronic Data Capture systems
Operations
- Patient registration
- Protocol management
- Data management
- Quality assurance
- Interim analysis
- Statistical analysis and interpretation
- Storage of clinical samples
- Trial registration at ClinicalTrials.gov
- Support for researchers writing papers
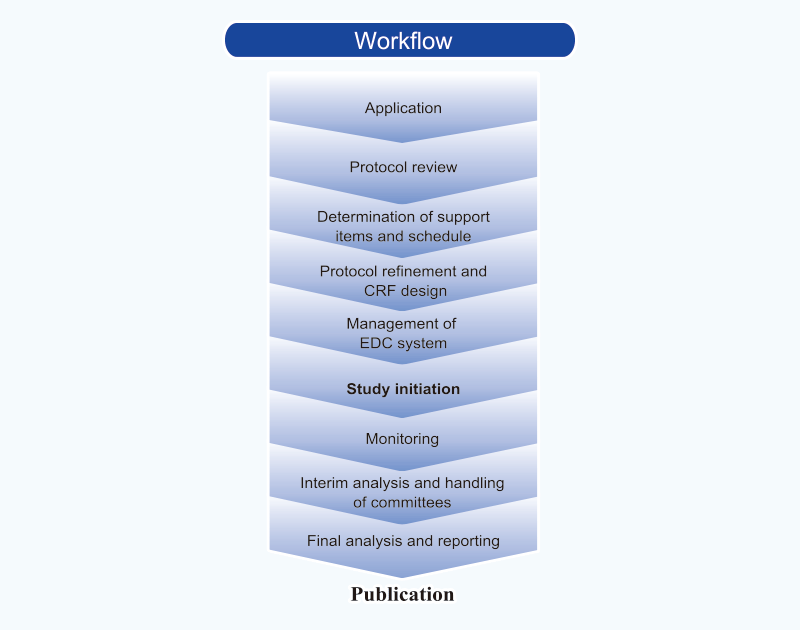
For further information about our clinical studies support services, please click here.
Disseminating medical and clinical research information
TRI is committed to keeping doctors and researchers informed of the latest in translational research. It does so through activities such as symposia and training sessions, as well by providing information to Japanese researchers about relevant guidelines and by translating international research information into Japanese.
- For example, TRI supports CDISC – an international not-for-profit that develops data standards to streamline clinical research and enable connections to healthcare world-wide. It does this by creating a website of the standards that is translated into Japanese.
TRI has sourced the most up-to-date and reliable therapeutic information available and translated it into Japanese online. For example, TRI has set up websites that follow various key topics and translate relevant information into Japanese. These websites include:
............Translated from the Physician Data Query (PDQ®) by the US National Cancer Institute
............Translated from the NCCN Guidelines® by the National Comprehensive Cancer Network
............Translated from the Alzheimer’s disease information page by the US National Institute on Aging
Request for donations and support
Our clinical information websites are kept up to date and freely accessible thanks to generous donations. If you are interested in supporting these efforts, please contact: