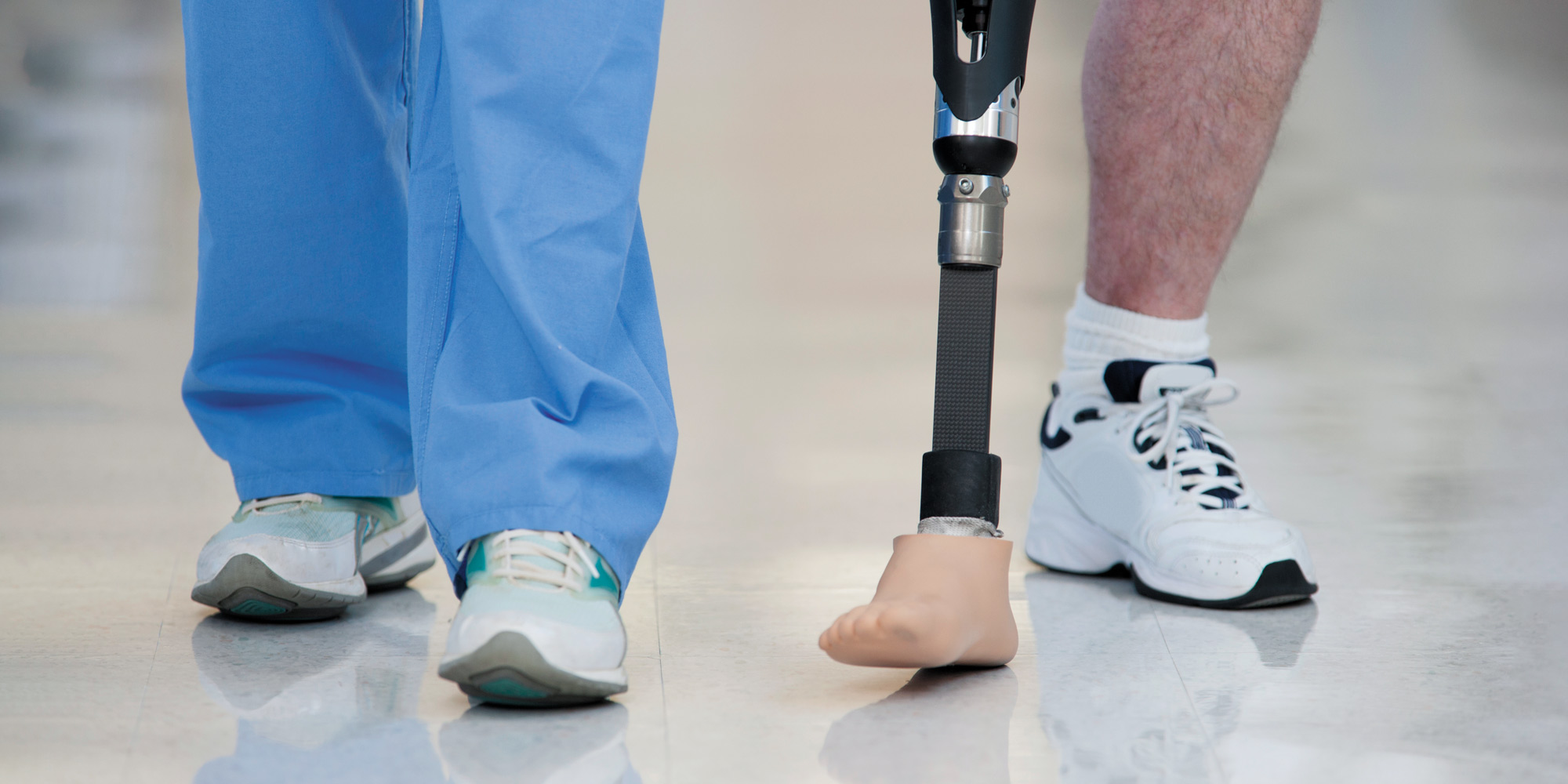
Patients with a serious circulatory disease in their legs who previously had no treatment options may soon be treated by using stem cells from their own peripheral blood.
Peripheral arterial disease is caused by narrowing or blockage of arteries in the limbs, and it afflicts an estimated 200 million plus people globally. The disease can develop into critical limb ischemia (CLI), which results in a damaging loss of blood supply in the lower extremities and often gives rise to non-healing ulcers or gangrene. This painful and dangerous condition frequently leads to amputation of a leg, and fewer than half of CLI patients live beyond 5 years.
Current treatments for CLI include pain control, treatment of ulcers and gangrene, and revascularization (including endovascular therapy or bypass surgery, when possible). However, a large proportion of patients cannot have revascularization because the vascular lesion is too extensive or they lack suitable veins for bypass grafts.
Yasuyuki Fujita and Atsuhiko Kawamoto at the Translational Research Center for Medical Innovation in Kobe, Japan, have reported how regenerative medicine using stem cells can potentially offer hope for these patients. Their description of the research appears in the TRI publication Principles of Regenerative Medicine.
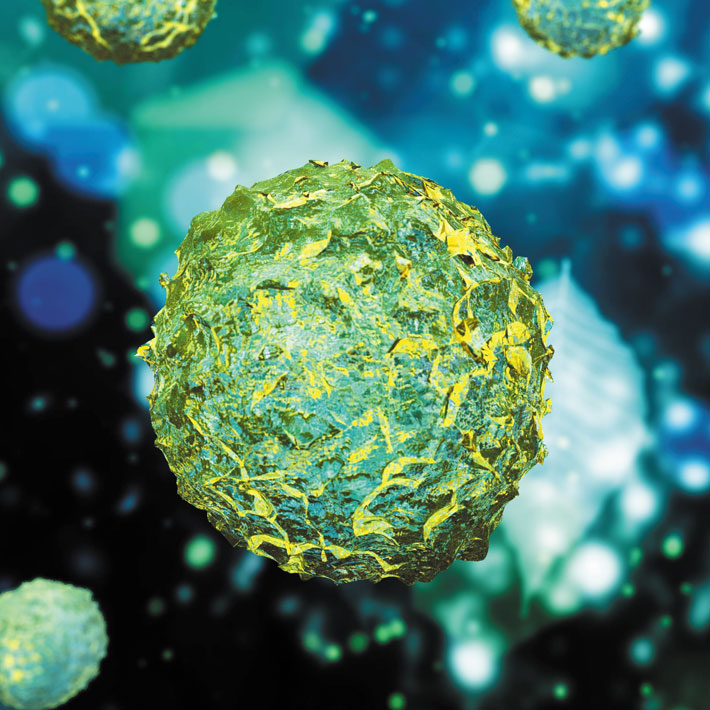
A class of stem cells known as endothelial progenitor cells (EPCs), a subset of CD34 antigen-positive (CD34+) cells of peripheral blood mononuclear cells, plays a role in regenerating the endothelium, the lining of blood vessels. EPCs are abundant in bone marrow and are released into the blood and carried to damaged tissue, where they help form new blood vessels.
Animal studies showed that transplanted EPCs integrated into new blood vessels in ischemic tissue to form the endothelium; EPCs also released various growth factors that promoted neovascular formation.
In a world-first, multicenter, single-blinded trial of neovascularization leg therapy in 2003, CD34+ cells were isolated from the granulocyte colony stimulating factor mobilized peripheral blood mononuclear cells of 17 CLI patients, purified and transplanted into the muscles of ischemic legs. One year after treatment, 88% of patients were free of CLI. Four years after treatment, the proportion free of CLI remained at more than 80%, and none of the patients had had a major amputation.
Similar positive results on efficacy and safety were achieved in a separate clinical study aimed at demonstrating a cell-sorting device for regulatory approval.
The cell-manufacturing process, which includes cell sorting under good gene, cellular, and tissue-based products manufacturing practice, has now been commissioned by the Foundation for Biomedical Research and Innovation at Kobe and a multicenter, randomized, comparative study is under way in order to gain approval of the CD34+ cells as a regenerative medicine product.
In 2018, this regenerative medicine product was designated by the Japanese government as appropriate for fast-track approval through the Sakigake strategy, which identifies medical devices, pharmaceuticals and regenerative medicine products that are designed to treat serious diseases and are likely to be developed first in Japan.
The approval of such a therapeutic cell product is expected to lead to its application in other ischemic diseases.