Author affiliations: *Department of Ophthalmology, Kyoto Prefectural University of Medicine; †Department of Ophthalmology, National Center for Geriatrics and Gerontology;‡Faculty of Life and Medical Sciences, Doshisha University; §Department for Medical Innovation and Translational Medical Science, Kyoto Prefectural University of Medicine; |Department of Frontier Medical Science and Technology for Ophthalmology, Kyoto Prefectural University of Medicine; ¶Foundation for Biomedical Research and Innovation at Kobe.
Severe ocular surface disorders such as Stevens–Johnson syndrome, ocular pemphigoid and thermal or chemical injury are difficult to treat and have a poorer visual prognosis than other stem cell deficiency conditions. Using an amniotic membrane as a substrate for culturing cells, researchers associated with the Translational Research Center for Medical Innovation (TRI) in Japan have generated stratified mucosal epithelial sheets consisting of corneal epithelial stem cells or autologous oral mucosal epithelial cells, and have used these to regenerate the ocular surface. Early clinical studies have demonstrated visual improvement for cicatricial corneal opacity, epithelial repair for refractory epithelial defect and efficacy for conjunctival sac reconstruction. The technique should be applicable to other diseases of the ocular surface.
1. INTRODUCTION
There are two types of mucosal epithelia on the ocular surface: the corneal epithelium and the conjunctival epithelium. The thin interface between the cornea and conjunctiva is called the limbus. The basal layer of the limbal epithelium is believed to contain corneal epithelial stem cells1–4. Corneal epithelial cells can no longer be supplied when the limbus is extensively damaged, which leads to the corneal surface being covered with the neighbouring conjunctival epithelium, fibrous tissue and blood vessels, severely impairing visual function. Diseases in which the function of corneal epithelial stem cells are impaired are known as limbal stem cell deficiency conditions3,5,6. Such conditions, which include Stevens–Johnson syndrome (SJS), ocular pemphigoid and thermal or chemical injury, are characterized by progressive fibrosis of subconjunctival connective tissues and inflammation. This leads to mucosal shrinkage or scarring or adhesion of the ocular surface, which produces severe dry eyes. SJS, ocular pemphigoid and thermal or chemical injury all involve cicatricial changes to the ocular surface and poor ocular surface conditions. These conditions have been classified as severe ocular surface disorders because they are especially severe, difficult to treat and have a poor visual prognosis compared to other stem cell deficiency conditions6,7.
Penetrating (or lamellar) keratoplasty, a common transplantation procedure, does not transplant limbal stem cells and thus it cannot be expected to give any clinical benefit for conditions involving stem cell deficiency. Transplantation of the mucosal epithelium, which contains stem cells, is required to treat stem cell deficiency. Hence, corneal epithelial transplantation procedures such as kerato-epithelioplasty and limbal transplantation have been developed8,9. However, epithelial transplantation requires a fresh donor, which is difficult to obtain. In addition, corneal epithelial transplantation has a higher rejection rate than penetrating keratoplasty. Studies have shown that persistent epithelial defects can easily occur after epithelial transplantation for severe ocular surface disorders. Even when an epithelial graft is accepted, progressive cicatricial changes can arise and persist in the long term, resulting in a poor visual prognosis7,10.
In 1997, Pellegrini et al. developed tissue-engineered corneal epithelial sheet transplantation as a novel therapeutic method for severe ocular surface disorders. They separated cells from a small amount of corneal–limbal epithelial tissue, generated epithelial sheets ex vivo, and then successfully transplanted them into two patients11. Since then, basic and clinical research on corneal regeneration has gained momentum worldwide.
We have focused on amniotic membrane as a substrate for culturing cells, generating stratified mucosal epithelial sheets consisting of corneal epithelial stem cells or oral mucosal epithelial cells, and using these to regenerate the ocular surface in severe ocular surface disorders12–14.
2. DEVELOPMENT OF NEW TREATMENT TECHNIQUES AND NON-CLINICAL PROOF OF CONCEPT
2.1 Clinical studies of transplantation of corneal epithelial sheets cultivated on amniotic membrane
The amniotic membrane is a thin membrane covering the fetal and placental surfaces. It consists of a basement membrane and a layer of amniotic epithelial cells. Because the amniotic membrane is unlikely to be rejected, it has long been used in surgery and dermatology. For example, it has been used to prevent adhesion after abdominal surgery and to promote epithelial repair of skin burns. The use of amniotic membrane in ophthalmology was reported in 1995 by Kim and Tseng, who used a cryopreserved amniotic membrane to reconstruct the ocular surface in rabbits15. Thereafter, amniotic membrane transplantation, with or without corneal epithelial transplantation, has been used to treat cicatricial diseases, including recurrent pterygium and SJS16.
The amniotic membrane is known to have various beneficial effects, including reducing scar formation, promoting epithelial growth mediated by growth factors17, reducing inflammation18 and reducing neovascularization. In particular, the collagen that makes up its basement membrane resembles that of the basement membrane of the ocular surface (i.e., corneal and conjunctival) epithelial cells19. Based on the assumption that selection of the substrate for culturing epithelial cells is important to obtain good prognosis, we established a method for culturing mucosal epithelial stem cells using a denuded amniotic membrane as the substrate. This was prepared by scraping off amniotic epithelial cells from cryopreserved amniotic tissue20–22.
In 1999, we began the clinical application of cultivated allogeneic corneal epithelial sheet transplantation for treating bilateral severe ocular surface disorders23,24. Cultivated corneal epithelial sheets were successfully grafted onto the cornea, and we were able to achieve epithelial reconstruction in patients having persistent epithelial defects with prolonged acute-phase inflammation, for which no other treatment was available. We also achieved visual improvement in patients with chronic visual impairment. Initially, we transplanted cultivated allogeneic corneal epithelial sheets in eyes with SJS, chemical injury or ocular pemphigoid (25 eyes in 23 patients). The transplanted corneal epithelium survived and maintained corneal transparency for a long time in patients with chemical injury. However, in patients with SJS and ocular pemphigoid the transplanted corneal epithelium was slowly replaced by conjunctival epithelium. Adverse events were obvious rejection (observed in four eyes) and corneal infection due to methicillin-resistant Staphylococcus aureus (MRSA) and Staphylococcus epidermidis (three eyes).
Most severe ocular surface diseases are bilateral, but in unilateral diseases, corneal epithelial sheets can be generated using cells taken from the other (healthy) eye. A patient with a unilateral chemical injury who received an autologous epithelial sheet transplant had a favorable outcome without developing complications25. Aiming for autologous transplantation in bilateral diseases, we started to develop cultivated epithelial sheets using patients’ own cells.
2.2 Transplantation of autologous oral mucosal epithelial sheets cultivated on amniotic membrane: establishing non-clinical proof of concept
We created cultivated oral mucosal epithelial sheets from rabbits and performed autologous transplantation of these sheets onto the ocular surface26. Rabbit oral mucosal epithelial cells cultivated on an amniotic membrane reached confluence in approximately 7 days, producing five to six layers of well-stratified epithelium, which was similar to corneal epithelium, in about 14 days. The surface layer had countless microvilli, and the cells were attached to each other by numerous desmosomal junctions. Hemidesmosome attachments were seen between the basal cells and the amniotic membrane. Immunohistology for keratins expressed in the cultivated oral mucosal epithelial sheets showed the presence of mucosa-specific keratin-4 and keratin-13 and the absence of epidermal-type keratin-1 and keratin-10, which was consistent with histological findings. Among corneal epithelial-specific keratins, keratin-3 was also present, but keratin-12 was not. These results allowed us to determine the histologic characteristics of the cultivated oral mucosal epithelial sheets: unlike the epidermis, which differentiates into keratinized cells, the cultivated oral mucosal epithelial cells had the characteristics of non-keratinized mucosa, while simultaneously expressing some cornea-specific keratins (keratin-3).
We created a corneal epithelial stem cell deficiency model. Briefly, after removing all corneal and conjunctival epithelial tissue up to 5 mm outside the limbus in the albino rabbits from which we collected oral mucosa, surrounding conjunctival tissue totally covered the ocular surface. After removing any conjunctival tissue covering the corneal surface in these eyes, we transplanted oral mucosal epithelial sheets that had been cultured on an amniotic membrane. Immediately after transplanting, the ocular surface showed a similar transparency to that observed following transplantation of cultivated corneal epithelial sheets; 48 hours after transplantation, we could confirm the survival of the cultivated epithelium. Ten days after transplantation, not only had the transplanted mucosal epithelial sheet survived on the ocular surface, but also fluorescein staining revealed that the epithelial cells had expanded outward from the mucosal sheet relative to 48 hours post-transplantation. These results confirmed that oral mucosal epithelial sheets cultivated on an amniotic membrane can survive and expand while maintaining corneal transparency on the ocular surface26.
Based on the data obtained from animal models, we began work on clinical application in humans. After obtaining adequate informed consent, we collected normal human oral mucosal tissue and attempted to determine specific conditions for creating human cultivated oral mucosal epithelial sheets. We found it was difficult to create human sheets under the same conditions used to create rabbit cultivated oral mucosal epithelial sheets, and we observed interspecies differences in cellular kinetics. However, we were able to facilitate the development of human cultivated oral mucosal epithelial sheets by modifying the culture process, including the culture medium27,28.
At almost at the same time, Nishida et al.29,30 developed another regenerative procedure using the patient’s own oral mucosal epithelial cells to cultivate and transplant autologous oral mucosal epithelial sheets. However, their method is very different as they transplant a sheet of epithelial cells fabricated on a temperature-responsive culture dish and do not use amniotic membrane. In our method, multilayered epithelial cells are attached to the base of the amniotic membrane, and thus are less likely to be lost by external stresses such as blinking or dryness.
With the aim of assessing the in vivo function of the human cultivated epithelial sheets, we performed xenogeneic transplantation of these sheets onto the ocular surface of rabbits. As with the rabbit model, the human cultivated oral mucosal epithelial sheet had similar morphologic features to corneal epithelium and survived on the ocular surface (Fig. 1)26,31.
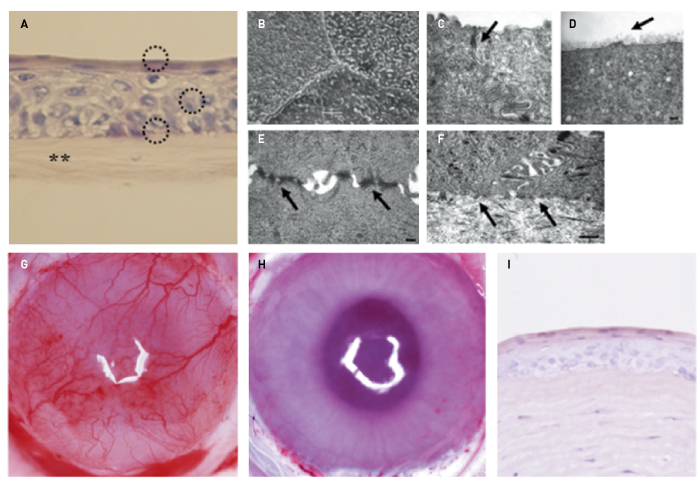
Figure 1. Establishment of non-clinical proof of concept. A, Rabbit oral mucosal epithelial cells cultivated on amniotic membrane (asterisks) produced four to five layers of cells (circles) that resembled normal corneal epithelium. B, D, Tight junctions were seen between the cells in the superficial layer (arrow in C). D, The most superficial layer was covered with glycocalyx-like material (arrow). E, The cells were attached to each other by many desmosomal junctions (arrows). F, The basal cells adhered to the amniotic membrane via hemidesmosome attachments (arrows). G, Before transplantation, the rabbit cornea showed stem cell loss and conjunctival invasion on the cornea. H, The oral mucosal epithelial sheet cultivated on amniotic membrane survived,expanded and maintained corneal transparency on the rabbit ocular surface (10 days after transplantation). I, The transplanted epithelial sheet attached to the corneal stroma without developing inflammatory cell infiltration or stromal edema.
B, C, D, E, F: © 2019 Association for Research in Vision and Ophthalmology (Modified from Ref. 26); A, G, H, I Reprinted from Ref. 14. with permission of Elsevier
3. SURGICAL PROCEDURES AND POSTOPERATIVE MANAGEMENT
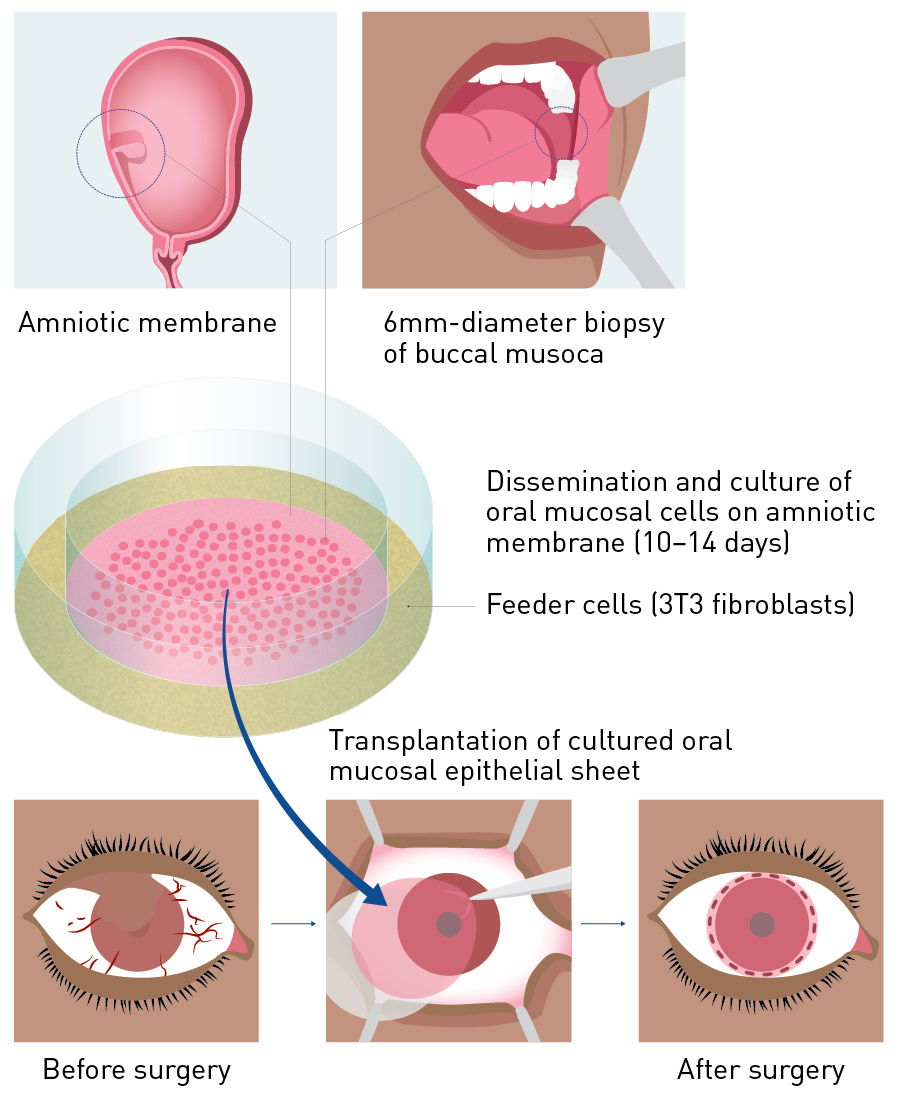
Figure 2. Procedure for transplanting cultivated autologous oral mucosal epithelial sheets. A mucosal specimen containing the oral mucosal epithelium was collected to create an oral mucosal epithelial sheet at the Cell Procession Center. After about 2 weeks, this stratified epithelial sheet was used for cultivated oral mucosal epithelial sheet transplantation.
From Ref. 41 © Kyoto Prefectural University of Medicine
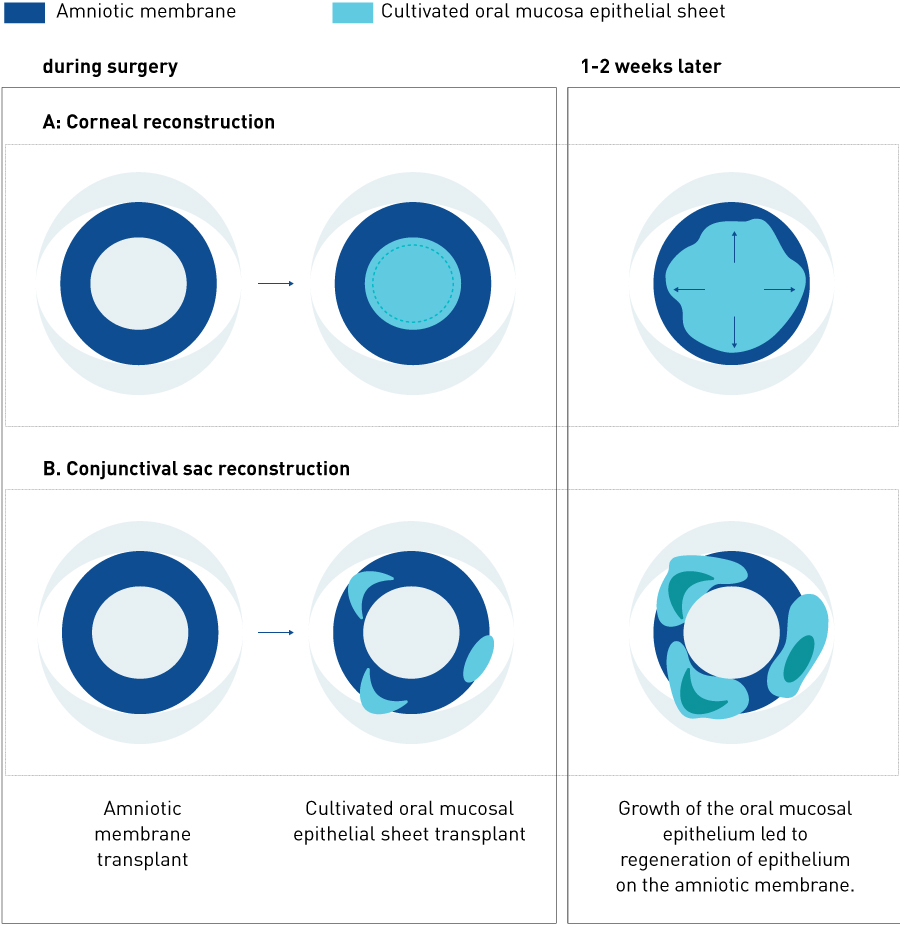
Figure 3. Extension of epithelial cells from the cultivated mucosal epithelial sheet after transplantation. A, Corneal reconstruction; B, conjunctival sac reconstruction.
Surgical procedures involve removing scar tissue from the ocular surface to expose the normal corneal stroma or sclera and transplanting the cultivated autologous oral mucosal epithelial sheet onto the cornea or sclera. The adhesion on the ocular surface is incised so as to release symblepharon and then subconjunctival proliferative tissues are removed. Any abnormal proliferative tissues present on the cornea and limbus are removed with a spatula to expose the corneal stroma. A microsponge soaked in 0.04% mitomycin C is applied to the periphery of the subconjunctival area for 4 minutes after removing subconjunctival connective tissues. The surgical area is then washed with approximately 300 ml of saline. The cultivated autologous oral mucosal epithelial sheet is sutured onto the cornea or sclera; this is combined with amniotic membrane transplantation in patients with widely exposed sclera (Fig. 2)31. When an amniotic membrane is transplanted, the oral mucosal epithelium extends smoothly from the transplanted oral mucosal epithelial sheet to the amniotic membrane (Fig. 3).
As the transplanted tissue is autologous, there is no need to use immunosuppressants to prevent rejection when transplanting cultivated oral mucosal epithelial sheets. However, in severe ocular surface diseases, particularly SJS and ocular pemphigoid, surgery induces significant inflammation, which induces epithelial disturbances and scarring of the ocular surface (e.g., symblepharon, which is conjunctivalization with fibrous tissue). It is thus critical to fully suppress inflammation of the ocular surface immediately after surgery. Since this is difficult to do with steroids alone, systemic immunosuppressants (cyclosporine) should be co-administered for cases with severe ocular surface scarring13,32. Ocular pemphigoid is a chronic, progressive, refractory autoimmune disease, and the use of cyclophosphamide can prevent disease progression.
Almost all patients also have dry eyes, and so artificial tears should be used frequently. The ocular surfaces of patients with severe ocular surface diseases, especially SJS, have an increased susceptibility to infection; particular attention should be paid to MRSA colonization and infection13,32. Preoperative assessment of the ocular surface and postoperative management are important factors that determine the prognosis after surgery.
4. THERAPEUTIC EFFECTS IN CLINICAL TRIALS AND INTERPRETATION
4.1 Overview
Since performing the world’s first successful transplantation of cultivated oral mucosal epithelial sheets in humans at Kyoto Prefectural University of Medicine in 200231, we have carefully collected cases of treatment of stem cell deficiency in refractory ocular conditions33,34. As the number of patients gradually increased, our project became an external seed translational research project within the Foundation for Biomedical Research and Innovation in 2008. With the support of the Translational Research Informatics Center, we conducted a retrospective study and statistical analysis of 72 patients (81 eyes, 86 surgeries) who had undergone transplantation of cultivated autologous oral mucosal epithelial sheets between June 2002 and December 200814,35–37.
Conditions that led to transplantation included SJS (21 patients), ocular pemphigoid (20 patients), thermal or chemical injury (13 patients), conjunctival malignancy (4 patients) and other conditions (14 patients). The purpose of treatment was broadly classified into four categories: visual improvement for cicatricial corneal opacity (47 transplants); epithelial repair for persistent epithelial defects with accompanying subacute inflammation (10 transplants); release of conjunctival sac adhesion (22 transplants); and other (7 transplants) (Fig. 4). The most common condition treated by corneal reconstruction (visual improvement and epithelial repair) was SJS, and the most common condition treated by conjunctival sac reconstruction was ocular pemphigoid.
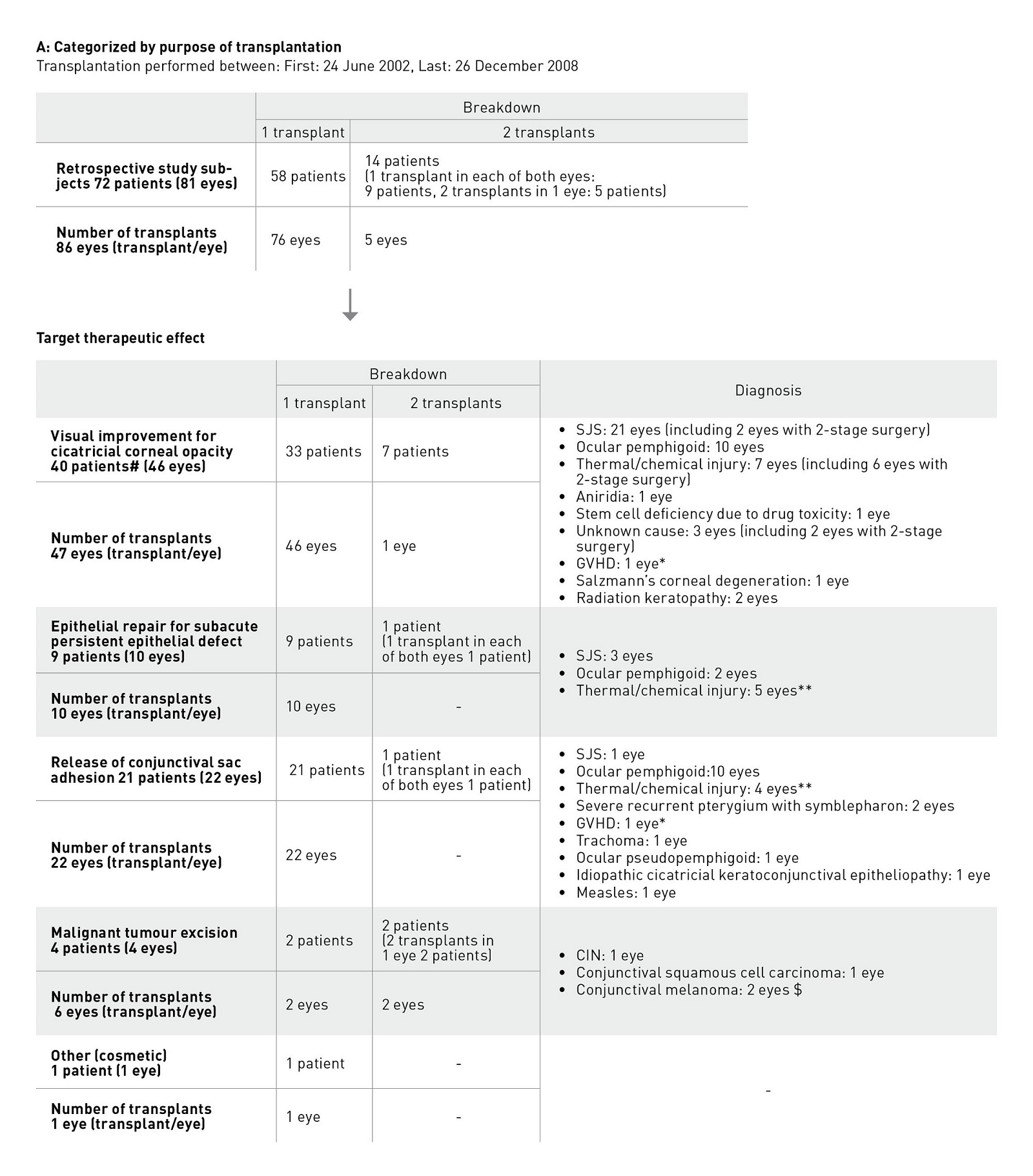
Figure 4. Patient details in early clinical studies. a, Categorized according to reason for transplantation; b, Categorized according to disease. CIN, conjunctival intraepithelial neoplasia ; GVHD, graft versus host disease; SJS, Stevens–Johnson syndrome #: Includes 1 patient in whom a transplant was also given to the other eye for a different purpose. *, **: Each includes 1 transplant performed on the same eye of the same patient for a different purpose. $: 2 transplants were given to the same eye of the same patient for the same purpose.
We examined visual acuity and ocular surface findings before transplantation, at weeks 4, 12 and 24 after transplantation, and at the final visit before the end of 2008, and assessed the success of the transplantation. We also noted all adverse events and used multivariate analysis to identify factors associated with their occurrence35,38.
4.2 Effects of epithelial sheet transplantation
Improvement in best-corrected visual acuity at the 24th week after surgery occurred in 46.8% of eyes in the visual improvement group; improvement in epithelial defect score was seen in 90.0% of eyes in the epithelial repair group; and improvement of symblepharon score was seen in 68.2% of eyes in the adhesion release group. Statistically significant efficacy was demonstrated in all groups.
4.2.1 Visual improvement
In total, 47 surgeries were performed in 46 eyes of 40 patients with the purpose of improving vision35. Conditions included SJS (21 eyes in 17 patients), ocular pemphigoid (10 eyes in 9 patients), thermal or chemical injury (7 eyes in 6 patients) and other (8 eyes in 8 patients), including idiopathic stem cell deficiency, radiation keratopathy, graft versus host disease (GVHD) and aniridia (Table 1, Fig. 4). Retransplantation was performed in one eye with radiation keratopathy.
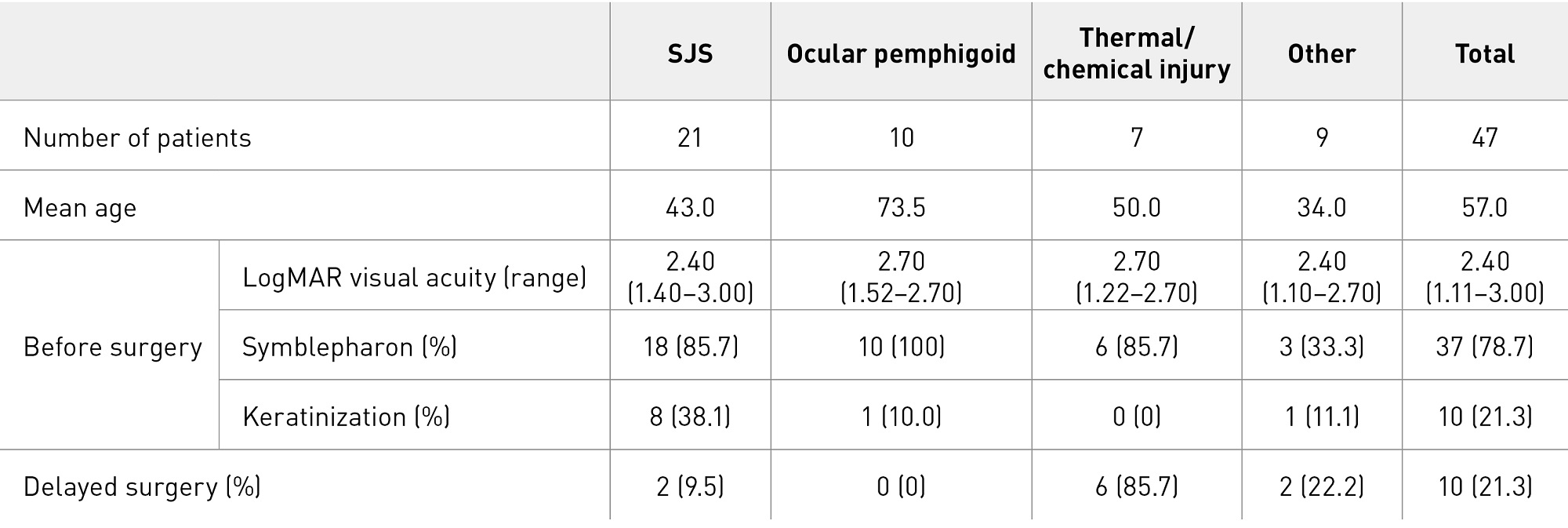
Table 1. Characteristics of patients treated for visual improvement.
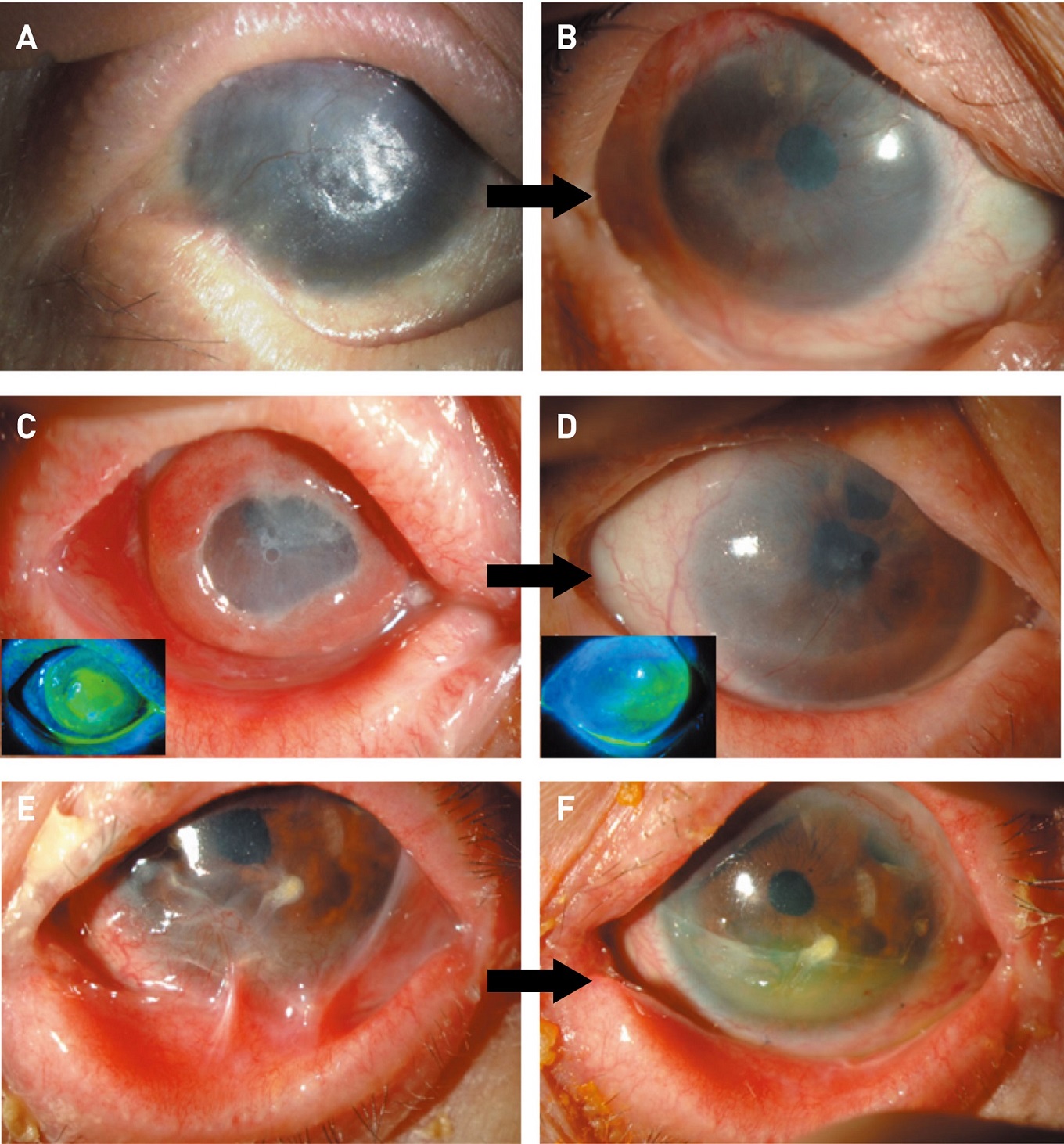
Figure 5. Representative cases. A, B, Epithelial sheet transplantation was performed 0.9 for visual improvement; C, D, for epithelial repair; E, F, for fornix reconstruction. Photographs were taken before surgery (A, C, E) and at the 24th postoperative week (B, D, F). (Modified from Refs. 32 and 34.)
A, B: Reprinted from Ref. 35 with permission from Elsevier; C, D: © 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
Improvement in best-corrected visual acuity at the 24th postoperative week was seen in 12 of 21 eyes with SJS (57.1%; 95% CI 34.0–78.1%), 3 of 10 eyes with ocular pemphigoid (30.0%; 95% CI 6.7–65.2%), and 3 of 7 eyes with thermal or chemical injury (42.9%; 95% CI 9.9–81.6%) (Fig. 5A and B). Patients with severe corneal stromal opacity underwent penetrating or lamellar keratoplasty after stabilizing the transplanted epithelium34. Six patients with thermal or chemical injury underwent this two-stage procedure and saw an improvement in their visual acuity after transplantation.
Cases with keratinization (abnormal differentiation of the epithelium) and symblepharon are the most serious of severe ocular surface diseases; they are also known as end-stage ocular surface diseases, and patients are usually ineligible for transplantation7. In the visual improvement group, preoperative symblepharon was observed in 37 eyes (78.7%) and keratinization in 10 eyes (21.3%), and the mean LogMAR visual acuity before surgery was 2.40 (i.e., counting fingers vision). If surgery can improve visual acuity below counting fingers to 0.01 or better, patients will be able to read or walk independently. We calculated the critical visual improvement rate as the proportion of patients whose preoperative vision was below counting fingers (best-corrected visual acuity of less than 0.01) and which had improved to 0.01 or better by the 24th postoperative week; the results were 50.0% (7 of 14 eyes), 42.9% (3 of 7 eyes) and 20.0% (1 of 5 eyes) for SJS, ocular pemphigoid and thermal or chemical injury, respectively. Eyes with thermal or chemical injury achieved visual improvement after delayed penetrating or lamellar keratoplasty. Improvement was seen in about half of the eyes with a severe disease that are usually considered unsuitable for surgery.
4.2.2 Epithelial repair
Epithelial repair was achieved in all patients with persistent epithelial defects and accompanying sub-acute inflammation (10 eyes in 9 patients [both eyes in 1 patient]), and the epithelial defect resolved within 4 weeks of transplantation in 7 eyes (Figs. 5C and D)38. Conditions treated included SJS (3 eyes), ocular pemphigoid (2 eyes) and thermal or chemical injury (5 eyes).
Persistent epithelial defect with accompanying sub-acute inflammation is a condition in which a non-healing epithelial defect is accompanied by persistent inflammation resulting from the development of a total corneal epithelial defect extending beyond the limbus during the acute phase of SJS or a thermal or chemical injury. This condition is resistant to all treatments, including eye drops and ointments, oral anti-inflammatory drugs and epithelial protection by eye patching or medical-use soft contact lenses. It carries a high risk of corneal infection, corneal stromal melting and corneal perforation, and may lead to blindness. Even if these complications are avoided, conjunctival and connective tissues eventually cover the corneal surface, leading to blindness. When cultivated mucosal epithelial sheet transplantation is used to treat this disease, epithelialization of the corneal surface is achieved at the time of surgery, which can help to prevent serious complications, such as infection and perforation. In addition, it can help inhibit postoperative inflammation of the ocular surface and reduce cicatricial changes6,38.
Transplantation of cultivated allogeneic corneal epithelial sheets produced a favorable outcome in treating this condition6,23. We obtained similar results with transplantation of cultivated autologous oral mucosal epithelial sheets.
4.2.3 Release of adhesion
Fornix reconstruction was performed in 22 eyes in 21 patients (both eyes for 1 patient) using cultivated autologous oral mucosal epithelial sheets for the purpose of adhesion release. The most common underlying condition was ocular pemphigoid (10 eyes), followed by thermal or chemical injury (4 eyes), SJS (1 eye) and other (7 eyes).
For the adhesion release group in ocular pemphigoid patients, the symblepharon score improved for 6 of 10 eyes, and the total upper and lower conjunctival sac adhesion score improved for 7 of 10 eyes (Figs. 5E and F). Cataract surgery involving transplanting epithelial sheets was performed in 5 eyes (50%) with ocular pemphigoid.
Ocular pemphigoid rarely presents with the acute symptoms seen in SJS or thermal or chemical injury. It develops without symptoms; shrinkage of the conjunctival sac progresses slowly over several years, and eventually leads to corneal epithelial stem cell deficiency and corneal opacity. Using transplantation of cultivated autologous oral mucosal epithelial sheets to reconstruct the conjunctival sac in ocular pemphigoid patients is significant because of its role as a rescue therapy, namely stem cell protection before the development of epithelial stem cell deficiency.
All patients with thermal or chemical injury saw an improvement in their adhesion score within 4 weeks of transplantation with no tendency towards recurrence thereafter. However, in the ocular pemphigoid group, improvement was maintained in 5 eyes (50%), but adhesion recurred in 4 eyes (40%). It is unclear whether the two different postoperative courses were due to host factors or environmental factors. Further investigation is needed to clarify the role of cultivated autologous oral mucosal epithelial sheet transplantation in ocular pemphigoid.
4.3 Adverse events
Adverse events included 24 cases of persistent epithelial defects in 22 patients (30.6%), 3 cases of infection in 3 patients (4.2%) and 7 cases of increased intraocular pressure due to steroids in 7 patients (9.7%). All cases of persistent epithelial defect ultimately healed, and none resulted in blindness.
Multivariate analysis to determine the factors associated with the occurrence of persistent epithelial defect in all cases of transplantation identified three factors: underlying disease (P = 0.0119), meniscus (P = 0.0323) and preoperative corneal neovascularization (P = 0.0806). These results indicate that an underlying condition of SJS, poor tear production with a very low meniscus and preoperative corneal neovascularization severe enough to cover the pupil are risk factors for developing persistent epithelial defect. In fact, persistent epithelial defect developed in 7 of 10 eyes having all three of these risk factors.
4.4 Long-term course
A long-term clinical follow-up of 19 eyes in 17 patients for over 3 years without additional intervention (including delayed surgery) revealed that the transplanted oral mucosal epithelium stabilized at 6 months after surgery and that a good epithelium was maintained on the ocular surface over a long period36.
4.5 Prospective clinical study
Based on the above results, we limited patients for a prospective clinical study to those with the three most severe refractory conditions (SJS, ocular pemphigoid and severe thermal or chemical injury) and performed transplantation of cultivated autologous oral mucosal epithelial sheets in 22 patients between September 2014 and March 2017 as part of a prospective clinical study under the Advanced Medical Care System37. The results are currently being analyzed.
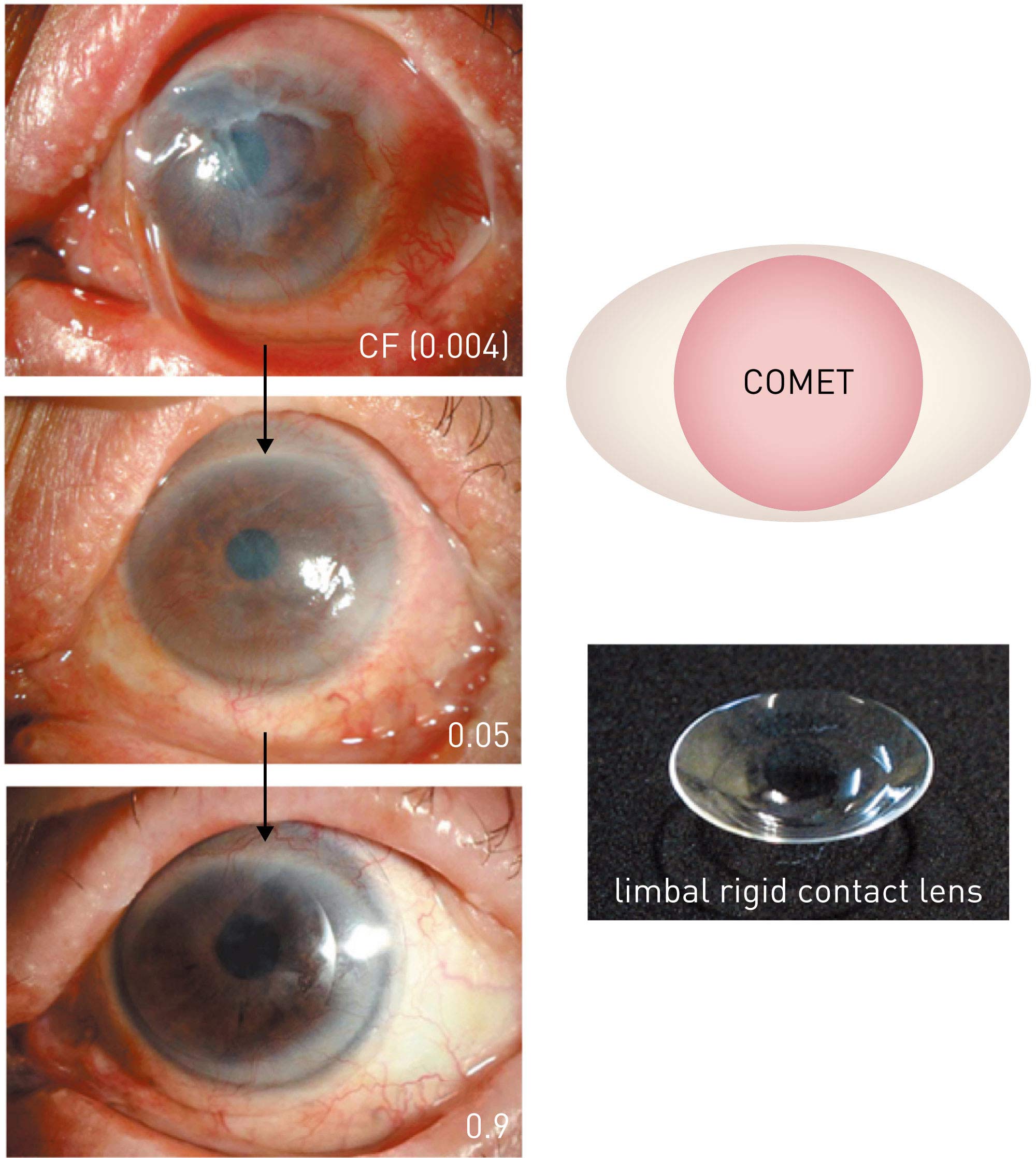
Figure 6. Improvement in visual function using limbal rigid contact lenses. A patient with Stevens–Johnson syndrome with severe adhesion on the ocular surface and a preoperative vision of counting fingers (0.004). The patient’s own oral mucosal epithelium transplanted onto the cornea was nearly stabilized 6 months after surgery,improving visual acuity to 0.05; the use of limbal rigid contact lenses further improved visual acuity to 0.9–1.0. This improvement has been maintained for over 7 years since surgery. (Modified from Ref. 43.) COMET, cultivated oral mucosal epithelial sheet transplantation
From Ref. 41 © 2019 Kyoto Prefectural University of Medicine
4.6 Visual rehabilitation using limbal contact lenses
With the aim of reducing irregular astigmatism and dry eyes in severe ocular surface diseases, we developed our own rigid contact lens (limbal rigid contact lens) design with a diameter of 13 to 14 mm. A clinical study demonstrated significant improvement in visual acuity and quality of life, particularly in SJS patients39. Therefore, we conducted an investigator-initiated study in SJS patients and obtained regulatory approval in February 201640.
Irregular astigmatism is present on the ocular surface after transplantation of cultivated autologous oral mucosal epithelial sheets. The use of limbal rigid contact lenses following epithelial stabilization after transplantation can reduce irregular astigmatism and improve visual function (Fig. 6)41. Limbal rigid contact lenses can increase the therapeutic effects of cultivated autologous oral mucosal epithelial sheet transplantation.
5. FUTURE PROSPECTS AND CHALLENGES
Transplantation of cultivated autologous oral mucosal epithelial sheets brings a ray of hope to vision-impaired patients with severe ocular surface diseases. The technique could be expanded to treat other conditions. However, both social and medical challenges need to be overcome in order to further improve outcomes.
5.1 Expansion of indications
SJS, ocular pemphigoid and thermal or chemical injury lead not only to loss of the corneal epithelium, but also to the loss of conjunctival epithelial stem cells. Diseases with similar conditions include GVHD and idiopathic stem cell deficiency, and future expansion to include these indications is expected.
Among the patients classified in the ‘Other’ category in our early clinical studies, 4 patients had malignant tumours. If transplantation of cultivated autologous oral mucosal epithelial sheets could be used to treat malignancy, it would allow us to take a sufficient safety margin during tumour excision and expect rapid epithelial repair after surgery.
5.2 Medical challenges
One study has reported the induction of differentiation of iPS cells into corneal epithelial cell-like cells, which may enable cultivated autologous corneal epithelial sheets to be transplanted for bilateral diseases in the future42. However, the morphology and nature of epithelial cells vary with transplantation site and environment, and the factors that control these changes are not fully understood. Transplanting cultivated autologous oral mucosal epithelial sheets had different outcomes depending on the underlying disease. We must continue our exploration of cell biology, including studying the cells in epithelial sheets and the relationship between the nature of the cells and their environment.
Recently, our group has developed a novel technique for culturing oral mucosal epithelial sheets using a feeder-free and serum-free culture system. Using this new protocol, we obtained favourable results in non-clinical studies43. A phase 3 clinical trial using the new culture protocol is currently being conducted.
5.3 Social challenges
Practical application of regenerative medicine requires a large investment in facilities, including cell-culture facilities; however, companies cannot expect to recoup their investments for rare diseases. Reducing visual impairment can reduce social security expenses (e.g., by removing the need for disability pensions) and increase the labour force, which greatly benefits society. However, reduced social security expenses are not reflected in corporate profits. Finding a balance between corporate profit and social benefit is critical for determining which problems should be addressed.
References
- Schermer, A., Galvin, S. & Sun, T. T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J. Cell Biol. 103, 49–62 (1986). | article
- Kinoshita, S., Kiritoshi, A., Ohji, M., Oohashi, S. & Manabe, R. Disappearance of palisades of Vogt in ocular surface diseases. Jap. J. Clin. Ophthal. 40, 363–366 (1986) [in Japanese]. | article
- Kinoshita, S., Adachi, W., Sotozono, C., Nishida, K., Yokoi, N. et al. Characteristics of the human ocular surface epithelium. Prog. Retin. Eye Res. 20, 639–673 (2001). | article
- Dua, H. S., Saini, J. S., Azuara-Blanco, A. & Gupta, P. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J. Ophthalmol. 48, 83–92 (2000). | article
- Tseng, S. C. G. Concept and application of limbal stem cells. Eye 3, 141–157 (1989). | article
- Kinoshita, S. Ocular surface reconstruction by tissue engineering. Nippon Ganka Gakkai Zasshi 106, 837–869 (2002) [in Japanese]. | article
- Holland, E. J. Epithelial transplantation for the management of severe ocular surface disease. Trans. Am. Ophthalmol. Soc. 94, 677–743 (1996). | article
- Thoft, R. A. Keratoepithelioplasty. Am. J. Ophthalmol. 97, 1–6 (1984). | article
- Turgeon, P. W., Nauheim, R. C., Roat, M. I., Stopak, S. S. & Thoft, R. A. Indications for keratoepithelioplasty. Arch. Ophthalmol. 108, 233–236 (1990). | article
- Thoft, R. A. & Sugar, J. Graft failure in keratoepithelioplasty. Cornea 12, 362–365 (1993). | article
- Pellegrini, G., Traverso, C. E., Franzi, A. T., Zingirian, M., Cancedda, R. et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 349, 990–993 (1997). | article
- Kinoshita, S., Sotozono, C., Inatomi, T., Nakamura, T., Koizumi, N. et al. Strategic research towards developing novel therapeutic methods for severe corneal diseases based on regenerative medicine. Saishinigaku 62, 132–180 (2007) [in Japanese]. | article
- Kinoshita, S. Future medical treatment for corneal diseases. Nippon Ganka Gakkai Zasshi 114, 161–199 (2010) [in Japanese]. | article
- Nakamura, T., Inatomi, T., Sotozono, C., Koizumi, N. & Kinoshita, S. Ocular surface reconstruction using stem cell and tissue engineering. Prog. Retin. Eye Res. 51, 187–207 (2016). | article
- Kim, J. C. & Tseng, S. C. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea 14, 473–484 (1995). | article
- Shimazaki, J., Yang, H. Y. & Tsubota, K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology 104, 2068–2076 (1997). | article
- Koizumi, N. J., Inatomi, T. J., Sotozono, C. J., Fullwood, N. J., Quantock, A. J. et al. Growth factor mRNA and protein in preserved human amniotic membrane. Curr. Eye Res. 20, 173–177 (2000). | article
- Ueta, M., Kweon, M. N., Sano, Y., Sotozono, C., Yamada, J. et al. Immunosuppressive properties of human amniotic membrane for mixed lymphocyte reaction. Clin. Exp. Immunol. 129, 464–470 (2002). | article
- Endo, K., Nakamura, T., Kawasaki, S. & Kinoshita, S. Human amniotic membrane, like corneal epithelial basement membrane, manifests the α5 chain of type IV collagen. Invest. Ophthalmol. Vis. Sci. 45, 1771–1774 (2004). | article
- Koizumi, N., Inatomi, T., Quantock, A. J., Fullwood, N. J., Dota, A. et al. Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea 19, 65–71 (2000). | article
- Koizumi, N., Fullwood, N. J., Bairaktaris, G., Inatomi, T., Kinoshita, S. et al. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest. Ophthalmol. Vis. Sci. 41, 2506–2513 (2000). | article
- Koizumi, N., Cooper, L. J., Fullwood, N. J., Inatomi, T., Kinoshita, S. et al. An evaluation of cultivated corneal limbal epithelial cells, using cell-suspension culture. Invest. Ophthalmol. Vis. Sci. 43, 2114–2121 (2002). | article
- Koizumi, N., Inatomi, T., Suzuki, T., Sotozono, C. & Kinoshita, S. Cultivated corneal epithelial transplantation for ocular surface reconstruction in acute phase of Stevens-Johnson syndrome. Arch. Ophthalmol. 119, 298–300 (2001). | article
- Koizumi, N., Inatomi, T., Suzuki, T., Sotozono, C. & Kinoshita, S. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology 108, 1569–1574 (2001) | article
- Nakamura, T., Inatomi, T., Sotozono, C., Koizumi, N. & Kinoshita, S. Successful primary culture and autologous transplantation of corneal limbal epithelial cells from minimal biopsy for unilateral severe ocular surface disease. Acta Ophthalmol. 82, 468–471 (2004). | article
- Nakamura, T., Endo, K., Cooper, L. J., Fullwood, N. J., Tanifuji, N. et al. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest. Ophthalmol. Vis. Sci. 44, 106–116 (2003). | article
- Nakamura, T. & Kinoshita, S. Ocular surface reconstruction using cultivated mucosal epithelial stem cells. Cornea 22, S75–S80 (2003). | article
- Kinoshita, S. & Nakamura, T. Development of cultivated mucosal epithelial sheet transplantation for ocular surface reconstruction. Artif. Organs. 28, 22–27 (2004). | article
- Hayashida, Y., Nishida, K., Yamato, M., Watanabe, K., Maeda, N. et al. Ocular surface reconstruction using autologous rabbit oral mucosal epithelial sheets fabricated ex vivo on a temperature-responsive culture surface. Invest. Ophthalmol. Vis. Sci. 46, 1632–1639 (2005). | article
- Nishida, K., Yamato, M., Hayashida, Y., Watanabe, K., Yamamoto, K. et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 351, 1187–1196 (2004). | article
- Nakamura, T., Inatomi, T., Sotozono, C., Amemiya, T., Kanamura, N. et al. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br. J. Ophthalmol. 88, 1280–1284 (2004). | article
- Sotozono, C., Inagaki, K., Fujita, A., Koizumi, N., Sano, Y. et al. Methicillin-resistant staphylococcus aureus and methicillin-resistant staphylococcus epidermidis infections in the cornea. Cornea 21, S94–S101 (2002). | article
- Inatomi, T., Nakamura, T., Koizumi, N., Sotozono, C., Yokoi, N. et al. Midterm results on ocular surface reconstruction using cultivated autologous oral mucosal epithelial transplantation. Am. J. Ophthalmol. 141, 267–275 (2006). | article
- Inatomi, T., Nakamura, T., Kojyo, M., Koizumi, N., Sotozono, C. et al. Ocular surface reconstruction with combination of cultivated autologous oral mucosal epithelial transplantation and penetrating keratoplasty. Am. J. Ophthalmol. 142, 757–764 (2006). | article
- Sotozono, C., Inatomi, T., Nakamura, T., Koizumi, N., Yokoi, N. et al. Visual improvement after cultivated oral mucosal epithelial transplantation. Ophthalmol. 120, 193–200 (2013). | article
- Nakamura, T., Takeda, K., Inatomi, T., Sotozono, C. & Kinoshita, S. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br. J. Ophthalmol. 95, 942–946 (2011). | article
- Sotozono, C., Inatomi, T., Nakamura, T., Koizumi, N., Hamuro, J. et al. Clinical study on cultivated autologous oral mucosal epithelial sheet transplantation for treating patients with intractable keratoconjunctival disease. Nippon Rinsho 73, 447–451 (2015) [in Japanese]. | article
- Sotozono, C., Inatomi, T., Nakamura, T., Koizumi, N., Yokoi, N. et al. Cultivated oral mucosal epithelial transplantation for persistent epithelial defect in severe ocular surface diseases with acute inflammatory activity. Acta Ophthalmol. 92, e447–e453 (2014). | article
- Sotozono, C., Yamauchi, N., Maeda, S. & Kinoshita, S. Tear exchangeable limbal rigid contact lens for ocular sequelae resulting from Stevens-Johnson syndrome or toxic epidermal necrolysis. Am. J. Ophthalmol. 158, 983–993 (2014). | article
- Sotozono, C. Clinical trial of tear exchangeable limbal supported rigid contact lens CS-100 for ocular sequelae due to Stevens-Johnson syndrome. Nippon Hyouka 43, 203–205 (2015) [in Japanese]. | article
- Sotozono, C. New treatments for practical refractory ocular surface disease and its practical application. J. Kyoto Pref. Univ. Med. 126, 145–155 (2017) [in Japanese]. | article
- Hayashi, R., Ishikawa, Y., Sasamoto, Y., Katori, R., Nomura, N. et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature 531, 376–380 (2016). | article
- Nakamura, T., Yokoo, S., Bentley, A. J., Nagata, M., Fullwood, N. J. et al. Development of functional human oral mucosal epithelial stem/progenitor cell sheets using a feeder-free and serum-free culture system for ocular surface reconstruction. Sci. Rep. 6, 37173 (2016). | article